2025-03-24 イリノイ大学アーバナ・シャンペーン校
<関連情報>
- https://aces.illinois.edu/news/new-ivf-method-mimics-fallopian-tube-environment-increasing-sperm-viability
- https://www.nature.com/articles/s41598-025-88986-2
- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0237666
精子と卵管との相互作用のモデルとして、ガラス表面に結合した卵管糖鎖に結合するブタ精子 Porcine sperm bind to an oviduct glycan coupled to glass surfaces as a model of sperm interaction with the oviduct
Sandra Soto-Heras,Larissa J. Volz,Nicolai Bovin & David J. Miller
Scientific Reports Published:08 February 2025
DOI:https://doi.org/10.1038/s41598-025-88986-2
Abstract
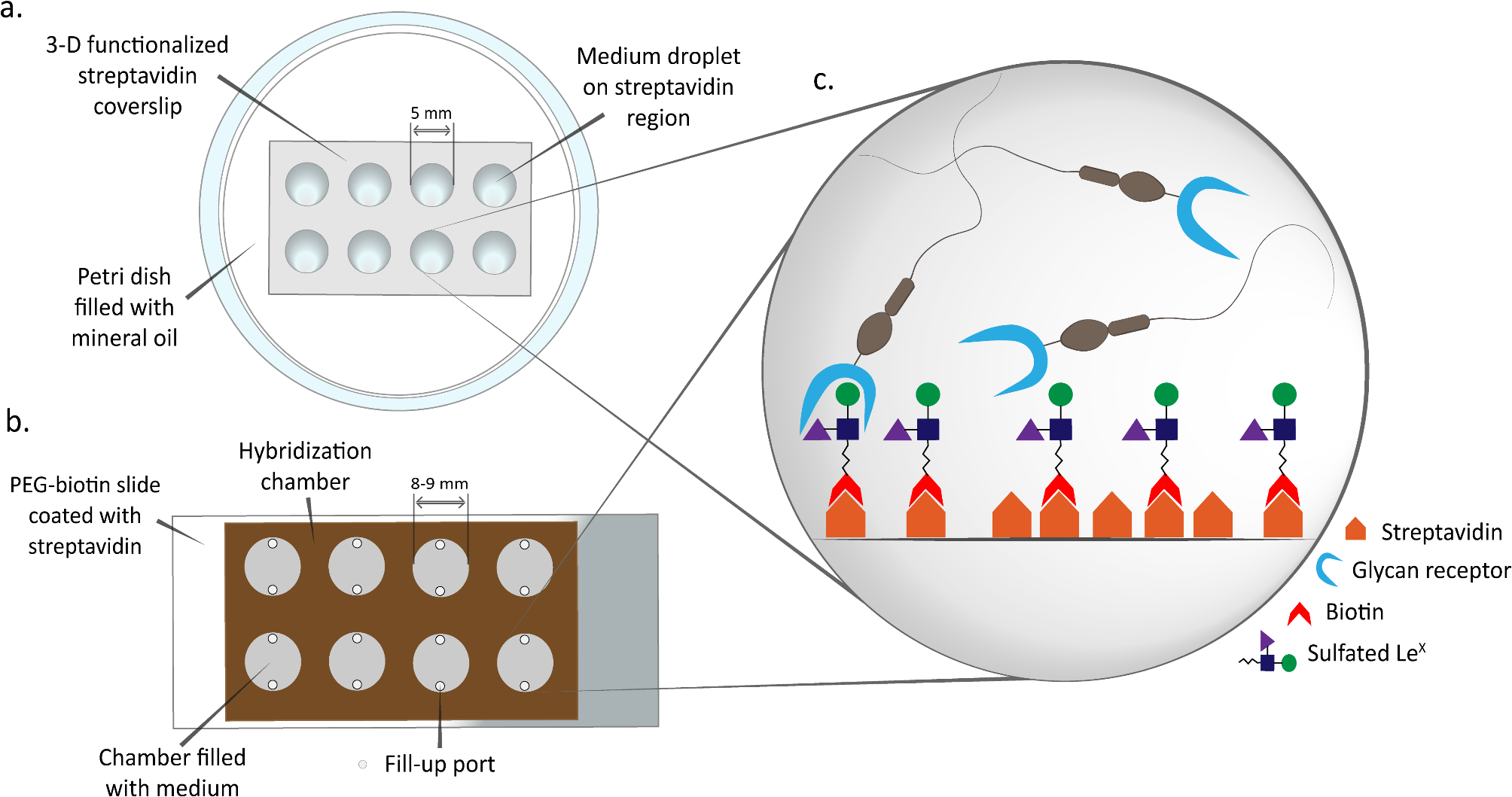
During transport through the oviduct, sperm interact with epithelial cells by attaching to specific glycans, a mechanism believed to select sperm and prolong their viability. An in vitro model of sperm-oviduct interactions was developed, consisting of a glass surface (either a slide or a coverslip) to which an oviduct glycan (sulfated Lewis X trisaccharide; suLeX) is coupled. The ability of porcine sperm to attach to suLeX-surfaces and detach in response to progesterone and mature cumulus-oocyte complexes (COCs) was validated. The suLeX-coverslip was adapted for in vitro fertilization (IVF), termed glycan-IVF, by allowing porcine sperm to first bind suLeX before transferring mature COCs. The glycan-IVF method produced a percentage of fertilized oocytes comparable to that of conventional IVF (75.1 vs. 72.0%). Finally, the ability of the suLeX-coverslip to maintain sperm fertilizing ability over time was assessed. After 24 h of incubation, fertilization by sperm bound to the suLeX-coverslip was sustained, compared to sperm with unmodified coverslips (12.0 vs. 1.0%, p < 0.05). Furthermore, the percentage of polyspermic zygotes was reduced in the suLeX-coverslip method (17.7 vs. 41.3%, p < 0.05). This study validated an in vitro model for studying sperm-oviduct interactions, with potential applications in assisted reproductive technologies.
卵管糖鎖への接着がブタ精子のCa2+流入と生存率を制御する Adhesion to oviduct glycans regulates porcine sperm Ca2+ influx and viability
Sergio A. Machado,Momal Sharif,Govindasamy Kadirvel,Nicolai Bovin,David J. Miller
PLOS One Published: August 21, 2020
DOI:https://doi.org/10.1371/journal.pone.0237666
Abstract
Before fertilization, sperm bind to epithelial cells of the oviduct isthmus to form a reservoir that regulates sperm viability and capacitation. The sperm reservoir maintains optimum fertility in species, like swine, in which semen deposition and ovulation may not be well synchronized. We demonstrated previously that porcine sperm bind to two oviductal glycan motifs, a biantennary 6-sialylated N-acetyllactosamine (bi-SiaLN) oligosaccharide and 3-O-sulfated Lewis X trisaccharide (suLeX). Here, we assessed the ability of these glycans to regulate sperm Ca2+ influx, capacitation and affect sperm lifespan. After 24 h, the viability of sperm bound to immobilized bi-SiaLN and suLeX was higher (46% and 41% respectively) compared to viability of free-swimming sperm (10–12%). Ca2+ is a central regulator of sperm function so we assessed whether oviduct glycans could affect the Ca2+ influx that occurs during capacitation. Using a fluorescent intracellular Ca2+ probe, we observed that both oviduct glycans suppressed the Ca2+ increase that occurs during capacitation. Thus, specific oviduct glycans can regulate intracellular Ca2+. Because the increase in intracellular Ca2+ was suppressed by oviduct glycans, we examined whether glycans affected capacitation, as determined by protein tyrosine phosphorylation and the ability to undergo a Ca2+ ionophore-induced acrosome reaction. We found no discernable suppression of capacitation in sperm bound to oviduct glycans. We also detected no effect of oviduct glycans on sperm motility during capacitation. In summary, LeX and bi-SiaLN glycan motifs found on oviduct oligosaccharides suppress the Ca2+ influx that occurs during capacitation and extend sperm lifespan but do not affect sperm capacitation or motility.