2025-04-11 東京大学
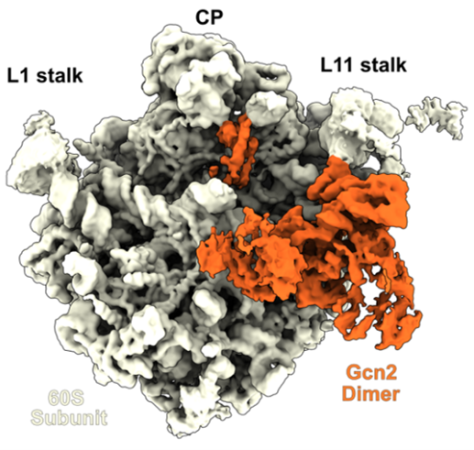
Gcn2-リボソーム大サブユニット複合体のクライオ電子顕微鏡構造
<関連情報>
- https://www.ims.u-tokyo.ac.jp/imsut/jp/about/press/page_00329.html
- https://www.ims.u-tokyo.ac.jp/imsut/content/000010895.pdf
- https://www.pnas.org/doi/10.1073/pnas.2415807122
Gcn2二量体と大型60Sリボソームサブユニットとの複合体の構造 Structure of a Gcn2 dimer in complex with the large 60S ribosomal subunit
Helge Paternoga, Lu Xia, Lyudmila Dimitrova-Paternoga, +9, and Daniel N. Wilson
Proceedings of the National Academy of Sciences Published:April 8, 2025
DOI:https://doi.org/10.1073/pnas.2415807122
Significance
Gcn2 is a critically important protein kinase of the integrated stress response (ISR) pathway and is conserved from yeast to humans. Although Gcn2 has long been known to interact with 60S subunits, structural insight into this interaction has been lacking. Here, we present a cryo-EM structure of a native yeast Gcn2–60S complex, revealing that Gcn2 is present as a dimer on the 60S subunit. We show that Gcn2 interaction with the 60S does not depend on the coactivators Gcn1 or Gcn20, and does not lead to activation of Gcn2. Rather interaction with the 60S subunit appears to maintain Gcn2 in a stand-by state, ready to dissociate and rebind ribosomes that stall and collide under stress conditions.
Abstract
The integrated stress response (ISR) is a central signaling network that enables eukaryotic cells to respond to a variety of different environmental stresses. Such stresses cause ribosome collisions that lead to activation of the kinase Gcn2, resulting in the phosphorylation and inactivation of eukaryotic initiation factor 2 and thereby promoting selective translation of mRNAs to restore homeostasis. Despite the importance of the ISR and intensive study over the past decades, structural insight into how Gcn2 interacts with ribosomal particles has been lacking. Using ex vivo affinity purification approaches, we have obtained a cryoelectron microscopy structure of a yeast Gcn2 dimer in complex with the ribosomal 60S subunit. The Gcn2 dimer is formed by dimerization of the histidine tRNA synthetase-like domains, which establish extensive interactions with the stalk-base and sarcin–ricin loop of the 60S subunit. The C-terminal domain of Gcn2 is also dimerized and occupies the A- and P-site tRNA binding sites at the peptidyl-transferase center of the 60S subunit. Complementary functional studies indicate that binding of Gcn2 to the 60S subunit does not require the coactivators Gcn1 or Gcn20, nor does it lead to phosphorylation of eIF2α. Instead, upon stress, we observe a shift of Gcn2 from the 60S subunit into the colliding ribosome fraction, suggesting that the Gcn2–60S complex represents an inactive stand-by state to enable a rapid redistribution to collided ribosomes, and thereby facilitating a quick and efficient response to stress.


