2025-04-16 カリフォルニア大学リバーサイド校(UCR)
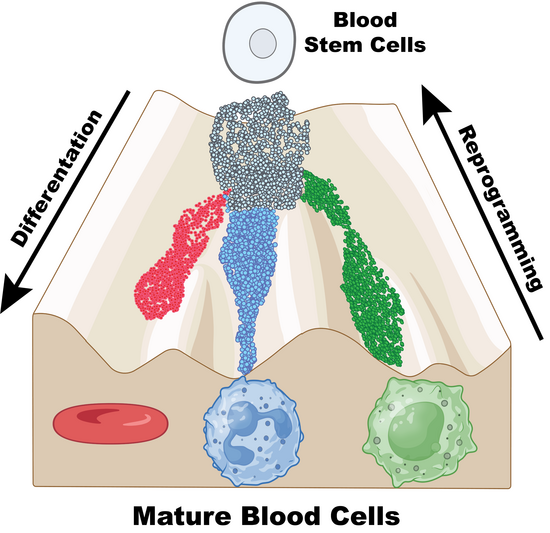
Stem cells (shown as small balls) can be imagined to roll down a landscape of hills and valleys, following specific paths as they mature (differentiation). Under normal conditions (blue), they stay on track. When histone chaperones stop working, the landscape shifts and cells may enter alternative valleys, taking on new fates (red or green). Through epigenetic reprogramming, cells can even be pushed back uphill or rerouted, changing their identity. Illustration is adapted from Waddington’s 1957 concept and created with BioRender. (UCR/B. Zhang)
<関連情報>
- https://news.ucr.edu/articles/2025/04/16/dna-organization-offers-clues-advancing-stem-cell-therapy
- https://genesdev.cshlp.org/content/early/2025/04/16/gad.352316.124.abstract
DNA複製と転写に結合したヒストンシャペロンが、分岐したクロマチンエレメントを制御し、細胞の運命を維持する Histone chaperones coupled to DNA replication and transcription control divergent chromatin elements to maintain cell fate
Reuben Franklin,Brian Zhang,Jonah Frazier,Meijuan Chen,Brian T. Do,Sally Padayao,Kun Wu,Matthew G. Vander Heiden,Christopher R. Vakoc,Jae-Seok Roe,Maria Ninova,Jernej Murn,David B. Sykes and Sihem Cheloufi
Genes & Development Published:April 16, 2025
DOI:10.1101/gad.352316.124
Abstract
The manipulation of DNA replication and transcription can be harnessed to control cell fate. Central to the regulation of these DNA-templated processes are histone chaperones, which in turn are emerging as cell fate regulators. Histone chaperones are a group of proteins with diverse functions that are primarily involved in escorting histones to assemble nucleosomes and maintain the chromatin landscape. Whether distinct histone chaperone pathways control cell fate and whether they function using related mechanisms remain unclear. To address this, we performed a screen to assess the requirement of diverse histone chaperones in the self-renewal of hematopoietic stem and progenitor cells. Remarkably, all candidates were required to maintain cell fate to differing extents, with no clear correlation with their specific histone partners or DNA-templated process. Among all the histone chaperones, the loss of the transcription-coupled histone chaperone SPT6 most strongly promoted differentiation, even more than the major replication-coupled chromatin assembly factor complex CAF-1. To directly compare how DNA replication- and transcription-coupled histone chaperones maintain stem cell self-renewal, we generated an isogenic dual-inducible system to perturb each pathway individually. We found that SPT6 and CAF-1 perturbations required cell division to induce differentiation but had distinct effects on cell cycle progression, chromatin accessibility, and lineage choice. CAF-1 depletion led to S-phase accumulation, increased heterochromatic accessibility (particularly at H3K27me3 sites), and aberrant multilineage gene expression. In contrast, SPT6 loss triggered cell cycle arrest, altered accessibility at promoter elements, and drove lineage-specific differentiation, which is in part influenced by AP-1 transcription factors. Thus, CAF-1 and SPT6 histone chaperones maintain cell fate through distinct mechanisms, highlighting how different chromatin assembly pathways can be leveraged to alter cell fate.

