新たな知見により、がん形成や抗がん剤治療への耐性に関する知見が深まることが期待される The new knowledge is expected to enhance our insight into cancer formation and resistance to antitumor therapy
2022-09-28 大韓民国・基礎科学研究院(IBS)
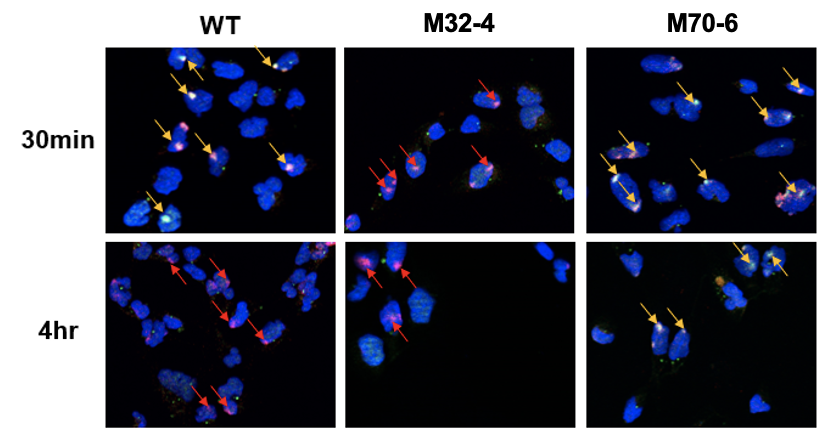
研究チームは、これらの相互作用について調査した。研究チームは、NERにおける2つの重要なタンパク質、すなわち色素性乾皮症タンパク質A(XPA)および複製タンパク質A(RPA)タンパク質が、NERにおけるプレインシジョン複合体の組織化に必要であることを発見した。
XPAとRPAの2つのタンパク質は、NERがDNAの損傷を発見した後の複合体の組織化に関与している。本研究では、これら2つのタンパク質の変異体を比較し、2つのタンパク質がNER経路にとって極めて重要な相互作用を行っていることを明らかにした。その結果、XPAとRPAの2つの相互作用界面がNERに重要であり、それぞれ異なる役割を担っていることが明らかになった。XPAとRPA32の相互作用はXPAがDNA損傷と最初に結合するのに重要であり、XPAとRPA70の相互作用はNERの完了に重要であることがわかった。
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=22071&pageIndex=1&searchCnd=&searchWrd=
- https://www.pnas.org/doi/10.1073/pnas.2207408119
ヌクレオチド除去修復におけるXPAとRPAの2つの相互作用面によるプレインシジョン複合体の組織化 Two interaction surfaces between XPA and RPA organize the preincision complex in nucleotide excision repair
Mihyun Kim https, Hyun-Suk Kim, Areetha D’Souza, Kaitlyn Gallagher, Eunwoo Jeong, Agnieszka Topolska-Woś, Kateryna Ogorodnik Le Meur, Chi-Lin Tsai, Miaw-Sheue Tsai, Minyong Kee, John A. Tainer, Jung-Eun Yeo, Walter J. Chazin and Orlando D. Schärer
Proceedings of National Academy of Sciences Published:August 15, 2022
DOI:https://doi.org/10.1073/pnas.2207408119
Significance
Nucleotide excision repair (NER) clears genomes of DNA adducts formed by UV light, environmental agents, and antitumor drugs. Defects in NER cause the skin cancer-prone disease xeroderma pigmentosum, yet NER also counteracts the efficacy of antitumor agents. NER operates through the stepwise assembly of the multiprotein complex and culminates in the excision of the DNA damage. We show that two interaction sites between the XPA and RPA proteins are critical for NER activity by structurally organizing the NER complex and licensing the DNA incision reactions. Our studies furthermore suggest that XPA and RPA constrict the DNA to assume a U-shape and place the two incision sites on the damaged DNA in close proximity to coordinate the two incision reactions.
Abstract
The xeroderma pigmentosum protein A (XPA) and replication protein A (RPA) proteins fulfill essential roles in the assembly of the preincision complex in the nucleotide excision repair (NER) pathway. We have previously characterized the two interaction sites, one between the XPA N-terminal (XPA-N) disordered domain and the RPA32 C-terminal domain (RPA32C), and the other with the XPA DNA binding domain (DBD) and the RPA70AB DBDs. Here, we show that XPA mutations that inhibit the physical interaction in either site reduce NER activity in biochemical and cellular systems. Combining mutations in the two sites leads to an additive inhibition of NER, implying that they fulfill distinct roles. Our data suggest a model in which the interaction between XPA-N and RPA32C is important for the initial association of XPA with NER complexes, while the interaction between XPA DBD and RPA70AB is needed for structural organization of the complex to license the dual incision reaction. Integrative structural models of complexes of XPA and RPA bound to single-stranded/double-stranded DNA (ss/dsDNA) junction substrates that mimic the NER bubble reveal key features of the architecture of XPA and RPA in the preincision complex. Most critical among these is that the shape of the NER bubble is far from colinear as depicted in current models, but rather the two strands of unwound DNA must assume a U-shape with the two ss/dsDNA junctions localized in close proximity. Our data suggest that the interaction between XPA and RPA70 is key for the organization of the NER preincision complex.