2025-04-17 カリフォルニア大学サンディエゴ校(UCSD)
<関連情報>
- https://today.ucsd.edu/story/key-enzyme-in-lipid-metabolism-linked-to-immune-system-aging
- https://link.springer.com/article/10.1007/s11357-025-01594-w
脂質のホメオスタシスにおける全身性の欠損が、加齢に伴うB細胞前駆細胞の発達障害を促進する Systemic deficits in lipid homeostasis promote aging-associated impairments in B cell progenitor development
Silvia Vicenzi,Fangyuan Gao,Parker Côté,Joshua D. Hartman,Lara C. Avsharian,Ashni A. Vora,R. Grant Rowe,Hojun Li,Dorota Skowronska-Krawczyk & Leslie A. Crews
GeroScience Published:15 April 2025
DOI:https://doi.org/10.1007/s11357-025-01594-w
Abstract
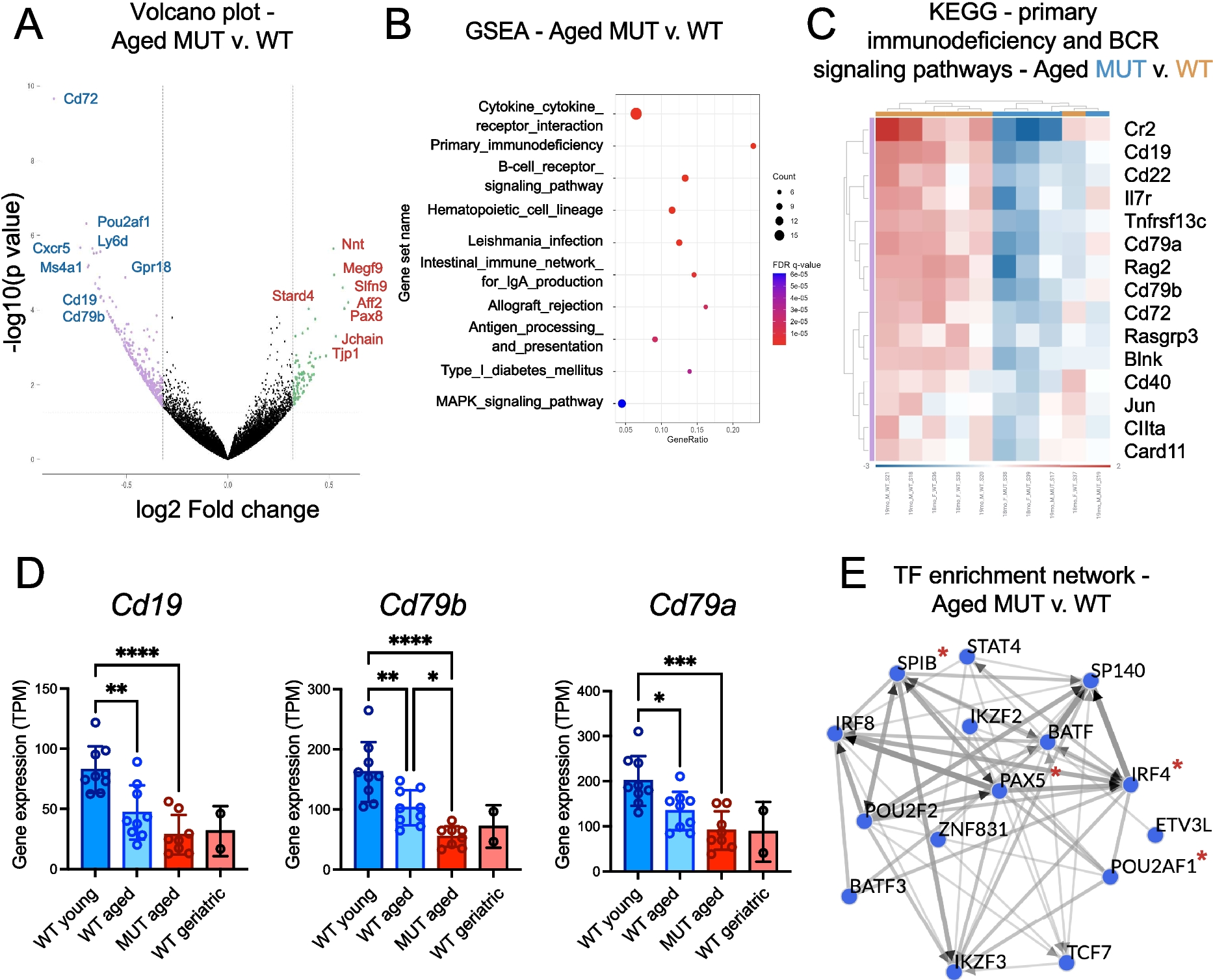
Organismal aging has been associated with diverse metabolic and functional changes across tissues. Within the immune system, key features of physiological hematopoietic cell aging include increased fat deposition in the bone marrow, impaired hematopoietic stem and progenitor cell (HSPC) function, and a propensity towards myeloid differentiation. This shift in lineage bias can lead to pre-malignant bone marrow conditions such as clonal hematopoiesis of indeterminate potential (CHIP) or clonal cytopenias of undetermined significance (CCUS), frequently setting the stage for subsequent development of age-related cancers in myeloid or lymphoid lineages. Human aging has also been associated with diverse lipid alterations across tissues, such as decreased phospholipid membrane fluidity that arises as a result of increased saturated fatty acid (FA) accumulation and a decay in n-3 polyunsaturated fatty acid (PUFA) species by the age of 80 years, however the extent to which impaired FA metabolism contributes to hematopoietic aging is less clear. Here, comprehensive multi-omics analyses uncovered a role for a key PUFA biosynthesis gene, ELOVL2, in mouse and human immune cell aging. Whole transcriptome RNA-sequencing studies and complementary flow cytometric analyses of bone marrow from aged Elovl2 mutant (enzyme-deficient) mice compared with age-matched controls revealed global downregulation in lymphoid cell markers and expression of genes involved specifically in B cell development. These studies unveiled CD79B, a vital molecular regulator of lymphoid progenitor development from the pro-B to pre-B cell stage, as a putative surface biomarker whose loss is associated with accelerated immune aging. The lipidome of mutant versus wild-type mice also displayed significant changes in the biophysical properties of cellular membranes. To investigate the relevance of these finding to human bone marrow aging, analyses of a single cell RNA-seq dataset of human HSPCs across the spectrum of human development and aging uncovered a rare subpopulation (< 7%) of CD34+ HSPCs that expresses ELOVL2 in healthy adult bone marrow. This HSPC subset, along with CD79B-expressing lymphoid-committed cells, were almost completely absent in CD34+ cells isolated from elderly bone marrow samples. Together, these findings uncover new roles for lipid metabolism enzymes in the molecular regulation of cellular aging and immune cell function in mouse and human hematopoiesis. In addition, because systemic loss of ELOVL2 enzymatic activity resulted in downregulation of B cell genes that are also associated with lymphoproliferative neoplasms, this study sheds light on an intriguing metabolic pathway that could be leveraged in future studies as a novel therapeutic modality to target blood cancers or other age-related conditions involving the B cell lineage.


