2025-04-25 ミュンヘン大学(LMU)
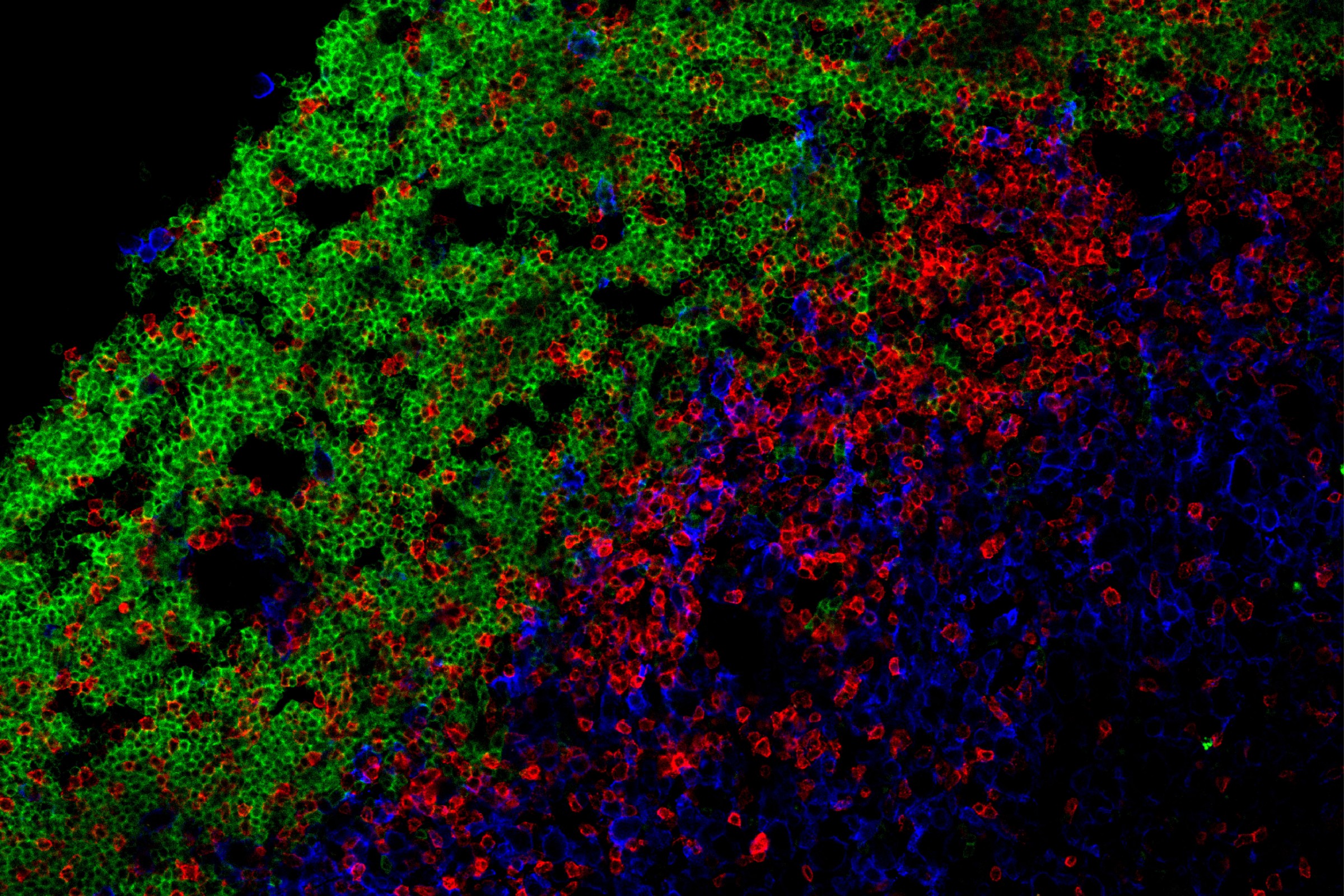
Accumulation of immune cells: B cells (green) and T cells (red) | © Peters Group
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/how-b-and-t-cells-fuel-the-pathological-process-in-ms.html
- https://www.science.org/doi/10.1126/sciimmunol.adn2784
異所性リンパ濾胞におけるT-B細胞の協力が中枢神経系自己免疫を伝播する T–B cell cooperation in ectopic lymphoid follicles propagates CNS autoimmunity
Anna Kolz, Clara de la Rosa, Isabel J. Syma, Sarah McGrath, […] , and Anneli Peters
Science Immunology Published:25 Apr 2025
DOI:https://doi.org/10.1126/sciimmunol.adn2784
Editor’s summary
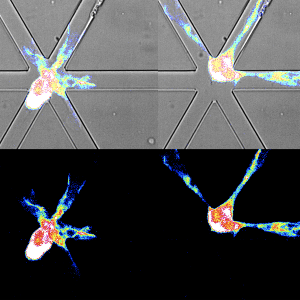
Ectopic lymphoid follicles (eLFs) are germinal center–like structures found in the meninges of some patients with multiple sclerosis (MS). eLFs could be a source of pathogenic T and B cells, but how these cells cooperate to drive MS pathogenesis is unclear. Kolz et al. used an EAE mouse model to study T–B cell interactions in eLFs. Using single-cell RNA sequencing, they identified distinct subpopulations of central nervous system (CNS) B cells. By intravital imaging of T–B cell interactions in eLFs, they show that T and B cells formed extensive contacts that were required to induce pathogenic cytokine effector profiles in autoreactive T cells. These findings suggest that T–B cell cooperation in eLFs promotes pathogenesis in MS. —Hannah Isles
Abstract
Meningeal ectopic lymphoid follicle (eLF)–like structures have been described in multiple sclerosis, but their role in central nervous system (CNS) autoimmunity is unclear. Here, we used a T helper 17 (TH17) adoptive transfer experimental autoimmune encephalomyelitis model featuring formation of eLFs. Single-cell RNA sequencing revealed that clusters of activated B cells and B1/marginal zone–like B cells were overrepresented in the CNS and identified B cells poised for undergoing germinal center reactions and clonal expansion in the CNS. Using intravital imaging to directly visualize TH17–B cell interactions, we demonstrated that T and B cells form long-lasting antigen-specific contacts in meningeal eLFs that result in reactivation of autoreactive T cells. CNS T cells depended on CNS B cells to maintain a proinflammatory cytokine profile. Our study reveals that extensive T–B cell cooperation occurs in meningeal eLFs, promoting both B cell differentiation and T cell reactivation, and may thereby propagate smoldering inflammation in the CNS.