2025-04-04 テキサス大学オースティン校(UT Austin)
 Aging and stress can induce cellular senescence in osteocytes, resulting in cytoskeletal and mechanical changes that impair their ability to sense mechanical signals, ultimately weakening bone.
Aging and stress can induce cellular senescence in osteocytes, resulting in cytoskeletal and mechanical changes that impair their ability to sense mechanical signals, ultimately weakening bone.
<関連情報>
- https://cockrell.utexas.edu/news/archive/10176-no-bones-about-it-new-details-about-skeletal-cell-aging-revealed
- https://onlinelibrary.wiley.com/doi/10.1002/smll.202408517
- https://onlinelibrary.wiley.com/doi/10.1111/acel.14421
骨における細胞老化の追跡:骨細胞の細胞骨格力学と形態学における時間依存的変化 Tracing Cellular Senescence in Bone: Time-Dependent Changes in Osteocyte Cytoskeleton Mechanics and Morphology
Maryam Tilton, Junhan Liao, Chanul Kim, Hossein Shaygani, Maria Astudillo Potes, Domenic J. Cordova, James L. Kirkland, Kyle M. Miller
Small Published: 03 March 2025
DOI:https://doi.org/10.1002/smll.202408517
Abstract
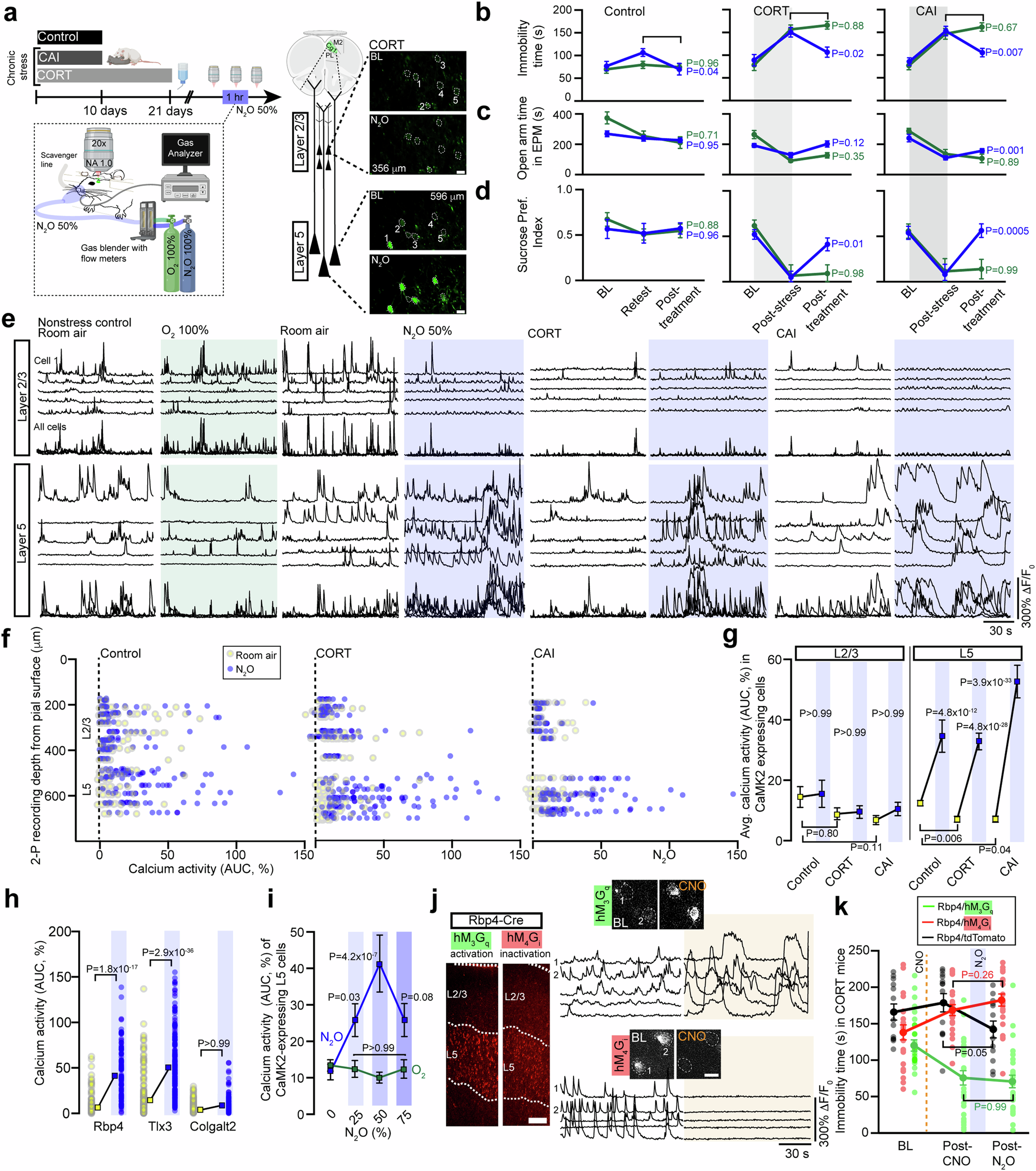
Aging-related bone loss significantly impacts the growing elderly population globally, leading to debilitating conditions such as osteoporosis. Senescent osteocytes play a crucial role in the aging process of bone. This longitudinal study examines the impact of continuous local and paracrine exposure to senescence-associated secretory phenotype (SASP) factors on biophysical and biomolecular markers in osteocytes. Significant cytoskeletal stiffening in irradiated (IR) osteocytes are found, accompanied by expansion of F-actin areas and a decline in dendritic integrity. These changes, correlating with alterations in pro-inflammatory cytokine levels and osteocyte-specific gene expression, support the reliability of biophysical markers for identifying senescent osteocytes. Notably, local accumulation of SASP factors have a more pronounced impact on osteocyte biophysical properties than paracrine effects, suggesting that the interplay between local and paracrine exposure can substantially influence cellular aging. This study underscores the importance of osteocyte mechanical and morphological properties as biophysical markers of senescence, highlighting their time dependence and differential effects of local and paracrine SASP exposure. Collectively, the investigation into biophysical senescence markers offers unique and reliable functional hallmarks for the non-invasive identification of senescent osteocytes, providing insights that can inform therapeutic strategies to mitigate aging-related bone loss.
老化の硬直化シンフォニー 老化骨細胞における生物物理学的変化 Stiffening symphony of aging: Biophysical changes in senescent osteocytes
Maryam Tilton, Megan Weivoda, Maria Astudillo Potes, Anne Gingery, Alan Y. Liu, Tamara Tchkonia, Lichun Lu, James L. Kirkland
Aging Cell Published: 24 November 2024
DOI:https://doi.org/10.1111/acel.14421
Abstract
Senescent osteocytes are key contributors to age-related bone loss and fragility; however, the impact of mechanobiological changes in these cells remains poorly understood. This study provides a novel analysis of these changes in primary osteocytes following irradiation-induced senescence. By integrating subcellular mechanical measurements with gene expression analyses, we identified significant, time-dependent alterations in the mechanical properties of senescent bone cells. Increases in classical markers such as SA-β-Gal activity and p16Ink4a expression levels confirmed the senescence status post-irradiation. Our key findings include a time-dependent increase in cytoskeletal Young’s modulus and altered viscoelastic properties of the plasma membrane, affecting the contractility of primary osteocytes. Additionally, we observed a significant increase in Sclerostin (Sost) expression 21 days post-irradiation. These biophysical changes may impair osteocyte mechanosensation and mechanotransduction, contributing to bone fragility. This is the first study to time-map senescence-associated mechanical changes in the osteocyte cytoskeleton. Our findings highlight the potential of biophysical markers as indicators of cellular senescence, providing more specificity than traditional, variable biomolecular markers. We believe these results may support biomechanical stimulation as a potential therapeutic strategy to rejuvenate aging osteocytes and enhance bone health.