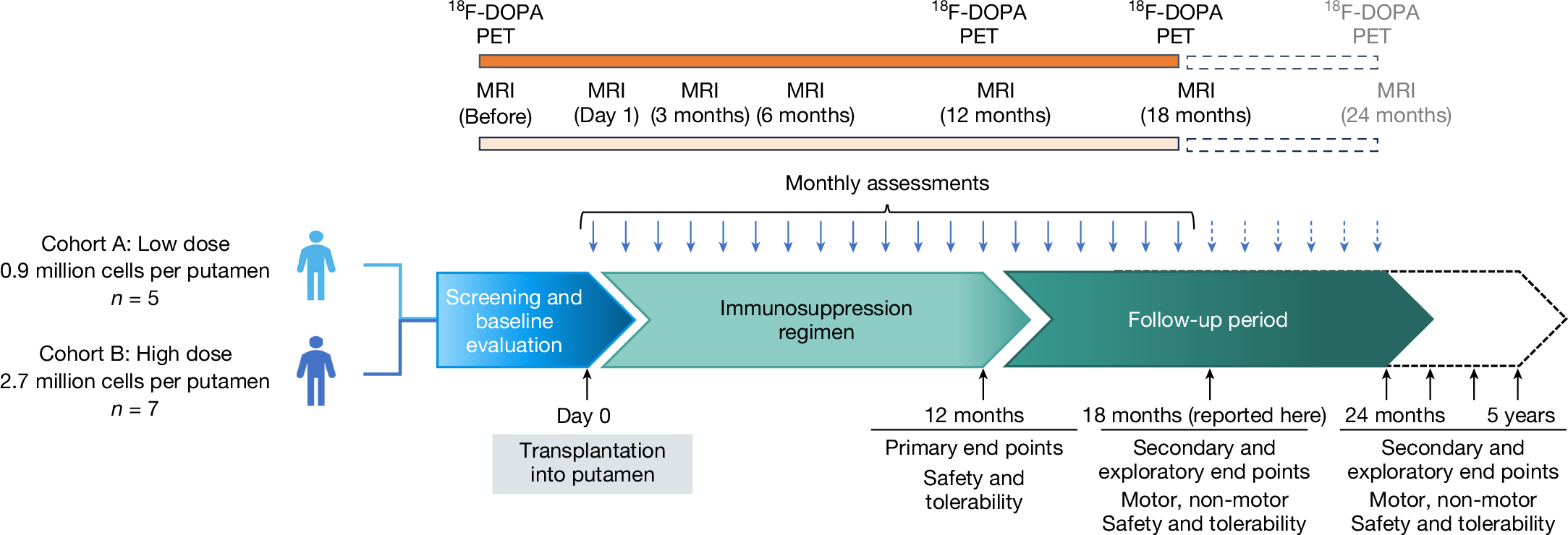
2025-05-08 カリフォルニア工科大学 (Caltech)
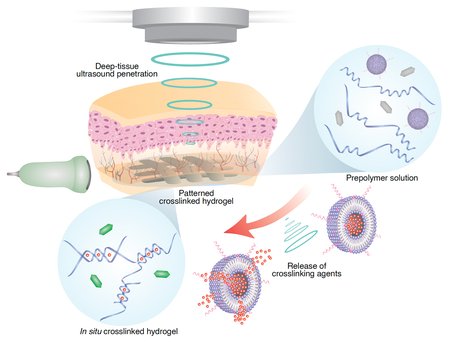
The deep tissue in vivo sound printing (DISP) platform. The technique combines ultrasound with low-temperature–sensitive liposomes loaded with crosslinking agents. The liposomes, often used for drug delivery, are embedded in a polymer solution containing the monomers of the desired polymer, an imaging contrast agent that reveals when crosslinking has occurred (here, the gas vesicles used for this purpose are shown as hexagons), and the cargo they hope to deliver—a therapeutic drug, for example. Scientists use focused ultrasound to increase the temperature in a targeted area by a few degrees, causing the liposomes to release their contents and initiate printing in a precise location.Credit: Elham Davoodi and Wei Gao
<関連情報>
- https://www.caltech.edu/about/news/3d-printing-in-vivo-using-sound
- https://www.science.org/doi/10.1126/science.adt0293
画像誘導による生体内深部組織のサウンドプリンティング Imaging-guided deep tissue in vivo sound printing
Elham Davoodi, Jiahong Li, Xiaotian Ma, Alireza Hasani Najafabadi, […] , and Wei Gao
Science Published:8 May 2025
DOI:https://doi.org/10.1126/science.adt0293
Editor’s summary
Three-dimensional (3D) printing is a valuable tool for generating patient-specific implants, either externally or even directly inside the body. The limitation of the former approach is the need for surgical implantation, whereas the latter approach is limited by the need for precursor materials and a polymerization method safe for in vivo use that can be activated with precision from outside of the body. Davoodi et al. developed a platform that uses imaging-guided ultrasound printing, which is capable of penetration depths much greater than the other approaches (see the Perspective by Kuang). The authors loaded cross-linking agents loaded into low-temperature–sensitive liposomes for incorporation into tunable bioinks. In vivo demonstrations included printing near diseased areas in a mouse bladder and deep within rabbit leg muscles. —Marc S. Lavine
Abstract
Three-dimensional printing offers promise for patient-specific implants and therapies but is often limited by the need for invasive surgical procedures. To address this, we developed an imaging-guided deep tissue in vivo sound printing (DISP) platform. By incorporating cross-linking agent–loaded low-temperature–sensitive liposomes into bioinks, DISP enables precise, rapid, on-demand cross-linking of diverse functional biomaterials using focused ultrasound. Gas vesicle–based ultrasound imaging provides real-time monitoring and allows for customized pattern creation in live animals. We validated DISP by successfully printing near diseased areas in the mouse bladder and deep within rabbit leg muscles in vivo, demonstrating its potential for localized drug delivery and tissue replacement. DISP’s ability to print conductive, drug-loaded, cell-laden, and bioadhesive biomaterials demonstrates its versatility for diverse biomedical applications.