2025-05-12 マウントサイナイ医療システム (MSHS)
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/mount-sinai-study-advances-understanding-of-personalized-vaccines-for-bladder-cancer
- https://www.nature.com/articles/s43018-025-00966-7
尿路上皮癌におけるアテゾリズマブと個別化新抗原ワクチン療法の併用:第1相試験 Atezolizumab plus personalized neoantigen vaccination in urothelial cancer: a phase 1 trial
Mansi Saxena,Jonathan F. Anker,Julia Kodysh,Timothy O’Donnell,Anna M. Kaminska,Marcia Meseck,Olivia Hapanowicz,Scot Anthony Niglio,Andres M. Salazar,Hardik R. Shah,Yayoi Kinoshita,Rachel Brody,Alex Rubinsteyn,Robert P. Sebra,Nina Bhardwaj & Matthew D. Galsky
Nature Cancer Published:09 May 2025
DOI:https://doi.org/10.1038/s43018-025-00966-7
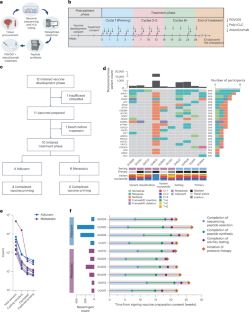
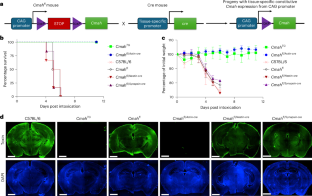
Abstract
Features of constrained adaptive immunity and high neoantigen burden have been correlated with response to immune checkpoint inhibitors (ICIs). In an attempt to stimulate antitumor immunity, we evaluated atezolizumab (anti-programmed cell death protein 1 ligand 1) in combination with PGV001, a personalized neoantigen vaccine, in participants with urothelial cancer. The primary endpoints were feasibility (as defined by neoantigen identification, peptide synthesis, vaccine production time and vaccine administration) and safety. Secondary endpoints included objective response rate, duration of response and progression-free survival for participants treated in the metastatic setting, time to progression for participants treated in the adjuvant setting, overall survival and vaccine-induced neoantigen-specific T cell immunity. A vaccine was successfully prepared (median 20.3 weeks) for 10 of 12 enrolled participants. All participants initiating treatment completed the priming cycle. The most common treatment-related adverse events were grade 1 injection site reactions, fatigue and fever. At a median follow-up of 39 months, three of four participants treated in the adjuvant setting were free of recurrence and two of five participants treated in the metastatic setting with measurable disease achieved an objective response. All participants demonstrated on-treatment emergence of neoantigen-specific T cell responses. Neoantigen vaccination plus ICI was feasible and safe, meeting its endpoints, and warrants further investigation. ClinicalTrials.gov registration: NCT03359239.