2025-05-13 中国科学院(CAS)
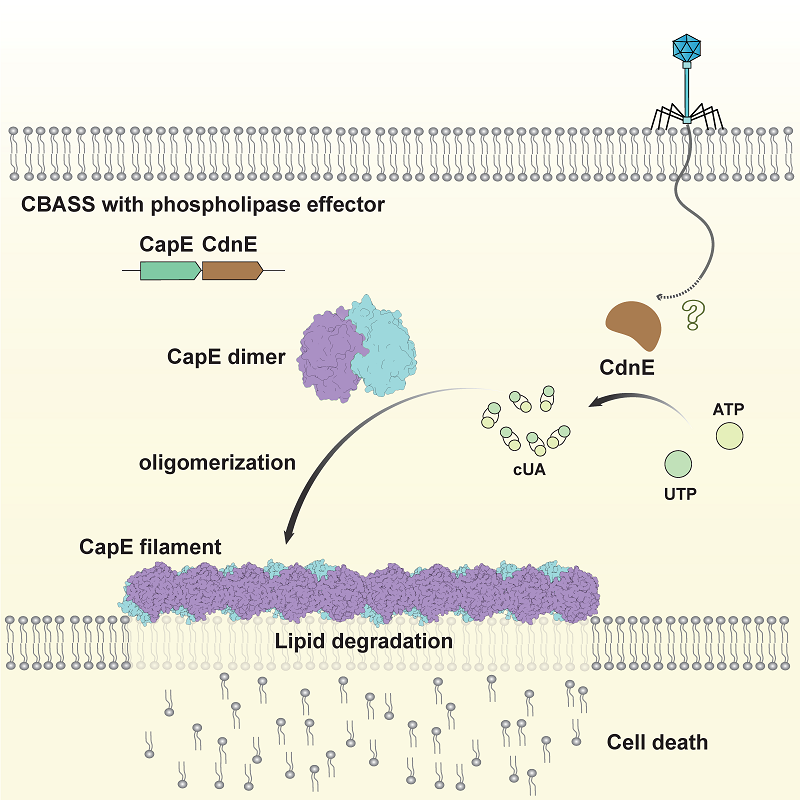
Model of anti-phage defense by CapE (Image by GAO Pu’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202505/t20250512_1042955.shtml
- https://www.cell.com/cell/abstract/S0092-8674(25)00457-X
環状ジヌクレオチドによって誘導されるホスホリパーゼのフィラメント状集合体は、広範なCBASS免疫を支配している Cyclic-dinucleotide-induced filamentous assembly of phospholipases governs broad CBASS immunity
Jingge Wang ∙ Zhao Li ∙ Hao Lang, ∙ … ∙ Dong Li, ∙ Pu Gao, ∙ Ang Gao
Cell Published:May 8, 2025
DOI:https://doi.org/10.1016/j.cell.2025.04.022
Highlights
- CBASS phospholipase effectors form inactive homodimers without ligand binding
- Cyclic dinucleotide binding induces filament formation of CBASS phospholipase effectors
- Filament assembly is crucial for phospholipase activation and membrane disruption
- Membrane disruption leads to cell lysis, preventing phage propagation
Summary
Cyclic-oligonucleotide-based antiphage signaling systems (CBASS), a widespread antiviral bacterial immune system homologous to the mammalian cGAS-STING pathway, synthesizes cyclic nucleotide signals and triggers effector proteins to induce cell death and prevent viral propagation. Among various CBASS effectors, phospholipase effectors are the first to be discovered and are one of the most widespread families that sense cyclic dinucleotides to degrade cell membrane phospholipids. Here, we report that CBASS phospholipases assemble from a dimeric inactive state into active higher-order filamentous oligomers upon sensing cyclic dinucleotides. Using a combined approach of cryo-electron microscopy and X-ray crystallography, we have determined the structures of CBASS phospholipase in the inactive dimeric state, the cyclic-dinucleotide-bound active higher-order state, and the substrate-analog-bound catalytic mimicry state, thereby visualizing the complete conformational reorganization process. Complemented by functional assays of intermolecular binding, phospholipase enzymatic activity, in vitro membrane disruption, and in vivo antiphage efficiency, our work elucidates the mechanisms of assembly and activation of CBASS phospholipases.