2025-05-15 中国科学院(CAS)
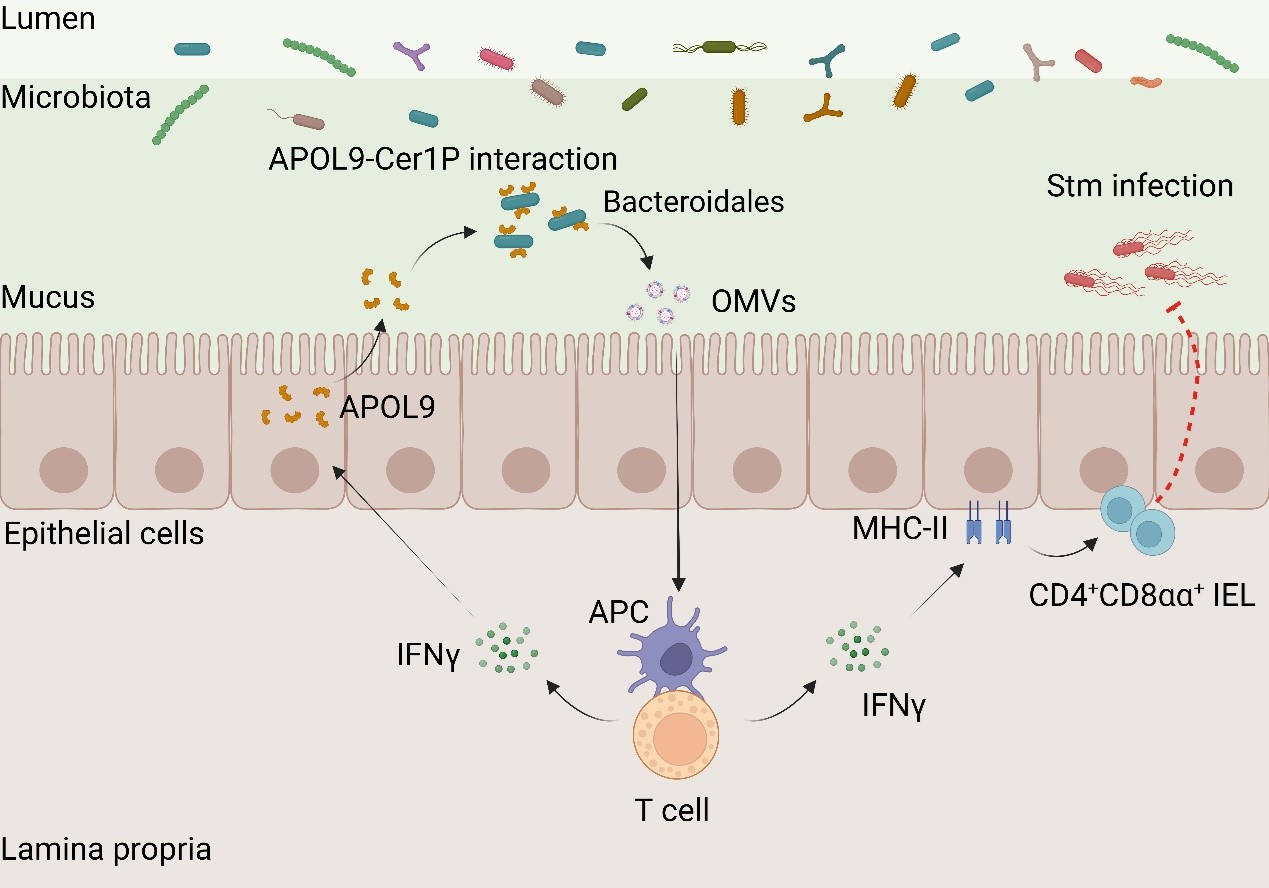
The proposed model for the novel apolipoprotein APOL9 that enhances mucosal immunity by activating the IFN-γ-MHC-II pathway through induction of OMVs release in Bacteroidales. (Image by Prof. QIAN’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202504/t20250429_1042385.shtml
- https://www.nature.com/articles/s41586-025-08990-4
アポリポタンパク質Lタンパク質による共生細菌の標的化が腸管免疫を調節する Targeting symbionts by apolipoprotein L proteins modulates gut immunity
Tao Yang,Xiaohu Hu,Fei Cao,Fenglin Yun,Kaiwen Jia,Mingxiang Zhang,Gaohui Kong,Biyu Nie,Yuexing Liu,Haohao Zhang,Xiaoyu Li,Hongyan Gao,Jiantao Shi,Guanxiang Liang,Guohong Hu,Dennis L. Kasper,Xinyang Song & Youcun Qian
Nature Published:14 May 2025
DOI:https://doi.org/10.1038/s41586-025-08990-4
Abstract
The mammalian gut harbours trillions of commensal bacteria that interact with their hosts through various bioactive molecules1,2. However, the mutualistic strategies that hosts evolve to benefit from these symbiotic relationships are largely unexplored. Here we report that mouse enterocytes secrete apolipoprotein L9a and b (APOL9a/b) in the presence of microbiota. By integrating flow cytometry sorting of APOL9-binding bacterial taxa with 16S ribosomal RNA gene sequencing (APOL9-seq), we identify that APOL9a/b, as well as their human equivalent APOL2, coat gut bacteria belonging to the order of Bacteroidales with a high degree of specificity through commensal ceramide-1-phosphate (Cer1P) lipids. Genetic abolition of ceramide-1-phosphate synthesis pathways in gut-dominant symbiote Bacteroides thetaiotaomicron significantly decreases the binding of APOL9a/b to the bacterium. Instead of lysing the bacterial cells, coating of APOL9a/b induces the production of outer membrane vesicles (OMVs) from the target bacteria. Subsequently, the Bacteroides-elicited outer membrane vesicles enhance the host’s interferon-γ signalling to promote major histocompatibility complex class II expression in the intestinal epithelial cells. In mice, the loss of Apol9a/b compromises the gut major histocompatibility complex class II-instructed immune barrier function, leading to early mortality from infection by intestinal pathogens. Our data show how a host-elicited factor benefits gut immunological homeostasis by selectively targeting commensal ceramide molecules.

