2025-05-20 北海道大学,大阪公立大学,東北大学,,扶桑薬品工業株式会社,株式会社ノースベッツ
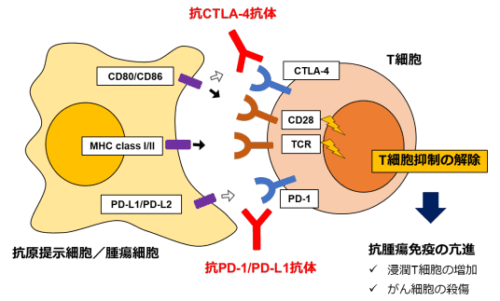
図 1. 免疫チェックポイント分子による腫瘍の免疫抑制メカニズムとその阻害剤による治療。 PD-1 及び CTLA-4 は T 細胞(免疫細胞)における抑制性受容体(免疫チェックポイント分子)であり、抗腫瘍免疫を低下させる。抗 PD-1/PD-L1 抗体及び抗 CTLA-4 抗体は免疫チェックポイント分子の機能を阻害することで抗腫瘍免疫を活性化させる。
<関連情報>
- https://www.hokudai.ac.jp/news/2025/05/ctla-4.html
- https://www.hokudai.ac.jp/news/pdf/250520_pr2.pdf
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1570717/full
抗PD-L1療法に耐性となったイヌ腫瘍における救援併用療法としてのイヌ化抗CTLA-4抗体の開発
Development of caninized anti-CTLA-4 antibody as salvage combination therapy for anti-PD-L1 refractory tumors in dogs
Naoya Maekawa,Satoru Konnai,Kei Watari,Hiroto Takeuchi,Takeshi Nakanishi,Taro Tachibana,Kenji Hosoya,Sangho Kim,Ryohei Kinoshita,Ryo Owaki,Madoka Yokokawa,Yumiko Kagawa,Satoshi Takagi,Tatsuya Deguchi,Hiroshi Ohta,Yukinari Kato,Satoshi Yamamoto,Keiichi Yamamoto,Yasuhiko Suzuki,Tomohiro Okagawa,Shiro Murata,Kazuhiko Ohash
Frontiers in Immunology Published:20 May 2025
DOI:https://doi.org/10.3389/fimmu.2025.1570717
Immune checkpoint inhibitors (ICIs) are widely used for cancer immunotherapy; however, the clinical efficacy of anti-PD-1/PD-L1 monotherapy is generally limited, highlighting the need to develop combination therapies. Dogs develop spontaneous tumors in immunocompetent settings, and anti-PD-1/PD-L1 antibodies exert similar clinical benefits. However, no clinically relevant anti-CTLA-4 antibody has been reported, limiting the value of canine tumors as comparative models for human ICI research. Here, canine CTLA-4 was molecularly characterized, and a caninized anti-CTLA-4 antibody (ca1C5) that blocks CTLA-4/ligand binding was developed. Treatment with ca1C5 increased cytokine production in canine immune cell cultures, and the immunostimulatory effect was enhanced when used in combination with the anti-PD-L1 antibody c4G12. As a proof-of-concept, a veterinary clinical study was conducted to demonstrate the safety and clinical efficacy of anti-CTLA-4 antibody as salvage combination therapy in dogs with advanced tumors refractory to prior c4G12 monotherapy. The combination treatment (c4G12 plus ca1C5) was well-tolerated, and evidence of antitumor activity was observed in one dog with oral malignant melanoma. Further studies are warranted to advance veterinary care for dogs and to better characterize canine ICI models for human onco-immunology research.