2025-06-03 マックス・プランク研究所(MPI)
Sage-leaved alangium (Alangium salviifolium) and ipecac (Carapichea ipecacuanha). © MPI f. Chemical Ecology/ Maite Colinas
<関連情報>
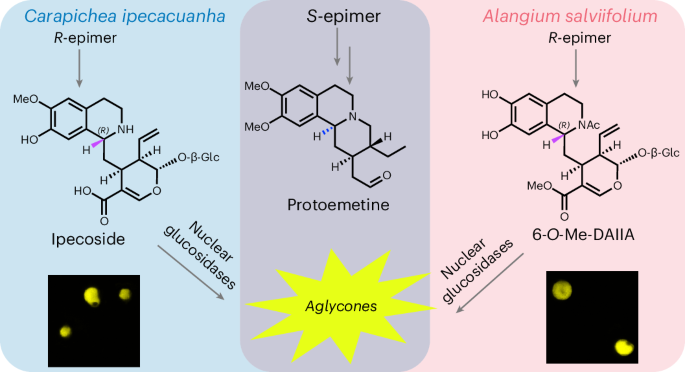
進化的に遠い2つの植物におけるイペカクアルカロイド生合成 Ipecac alkaloid biosynthesis in two evolutionarily distant plants
Maite Colinas,Clara Morweiser,Olivia Dittberner,Bianca Chioca,Ryan Alam,Helena Leucke,Yoko Nakamura,Delia Ayled Serna Guerrero,Sarah Heinicke,Maritta Kunert,Jens Wurlitzer,Kerstin Ploss,Benke Hong,Veit Grabe,Adriana A. Lopes & Sarah E. O’Connor
Nature Chemical biology Published:03 June 2025
DOI:https://doi.org/10.1038/s41589-025-01926-z
A preprint version of the article is available at bioRxiv.
Abstract
Ipecac alkaloids are medicinal monoterpenoid-derived tetrahydroisoquinoline alkaloids found in two distantly related plants: Carapichea ipecacuanha (Gentianales) and Alangium salviifolium (Cornales). Here we provide evidence suggesting that both plants initiate ipecac alkaloid biosynthesis through a nonenzymatic Pictet–Spengler reaction and we elucidate the biosynthetic fate of both the 1R and 1S stereoisomers that are produced in this nonstereoselective reaction. Although the biosynthesis of the 1S-derived protoemetine proceeds according to the same chemical logic in both species, each plant uses a distinct monoterpene precursor. Phylogenetic analyses show examples of independent pathway evolution through parallel and convergently evolved enzymes. This work provides insight into how nature can capitalize on highly reactive starting substrates and the manner in which multistep pathways can arise and lays the foundation for metabolic engineering of these important medicinal compounds.