2025-06-04 アメリカ国立衛生研究所(NIH)
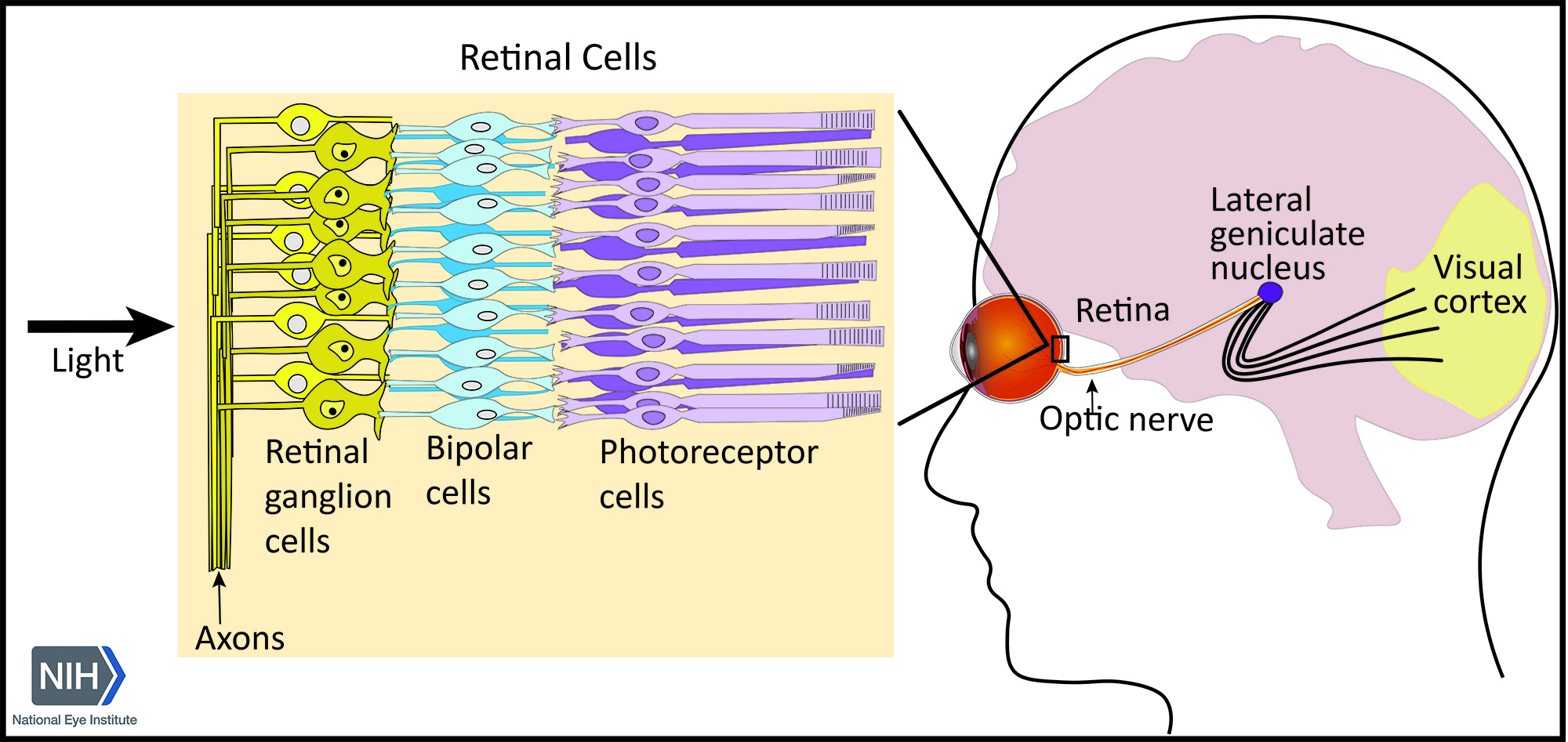
Visual processing involves interactions between neurons in the eye and brain allowing us to see the world around us. These pathways originate in the retina, which converts light energy into electrical signals that are transmitted to the brain’s visual processing centers. Axons from retinal ganglion cells form the optic nerve, which connects to the lateral geniculate nucleus of the thalamus, a relay center in the brain that transmits signals to the visual cortex – a part of the brain that processes those signals into images.
<関連情報>
- https://www.nih.gov/nih-researchers-identify-brain-circuits-responsible-visual-acuity
- https://www.jneurosci.org/content/45/23/e0436252025
網膜病変がLGN X細胞とY細胞の視覚反応に及ぼす影響の違い Differential Impact of Retinal Lesions on Visual Responses of LGN X and Y Cells
Jingyi Yang, Krystel Huxlin and Farran Briggs
Journal of Neuroscience Published:4 June 2025
DOI:https://doi.org/10.1523/JNEUROSCI.0436-25.2025
Abstract
Damage to retinal cells from disease or injury causes vision loss and remodeling of downstream visual information processing circuits. As retinal cell replacement therapies and prosthetics become increasingly viable, we must understand the postretinal consequences of retinal cell loss to optimally recover visual perception. Here, we asked whether loss of retinal ganglion cells (RGCs) differentially impacts postsynaptic neurons in the visual thalamus—the dorsal lateral geniculate nucleus (LGN)—of ferrets, highly visual carnivores. We hypothesized that RGC loss might impact X more than Y LGN neurons, as there is less divergence in X retinogeniculate connections. We induced excitotoxic lesions of RGCs in a single eye and recorded neurophysiological responses of both contra- and ipsilesional LGN neurons to a variety of visual stimuli. We observed loss of responses among many LGN neurons, presumably with receptive fields within the scotoma. We also observed contralesional LGN neurons with receptive fields within or at the border of the scotoma that responded consistently to drifting sinusoidal gratings and spatiotemporally dynamic stimuli, enabling their classification as X or Y cells. Contralesional Y cell responses remained intact while contralesional X cells demonstrated higher firing rates, altered tuning to stimulus contrast and temporal frequency, and reduced spike timing precision. Consistent with neurophysiological results, alpha RGCs appeared relatively spared compared with beta RGCs. Together, our findings show that retinal cell loss differentially impacts downstream neuronal circuits, suggesting that supplemental vision recovery therapies may need to target visual circuits specialized for acuity vision.


