2025-06-05 ロックフェラー大学
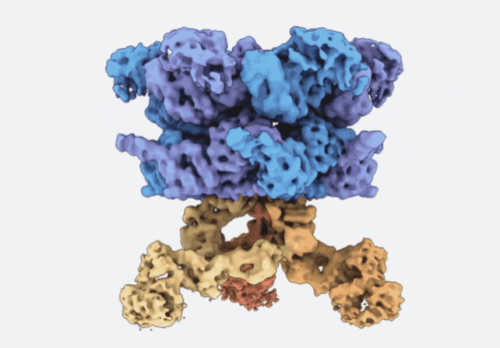
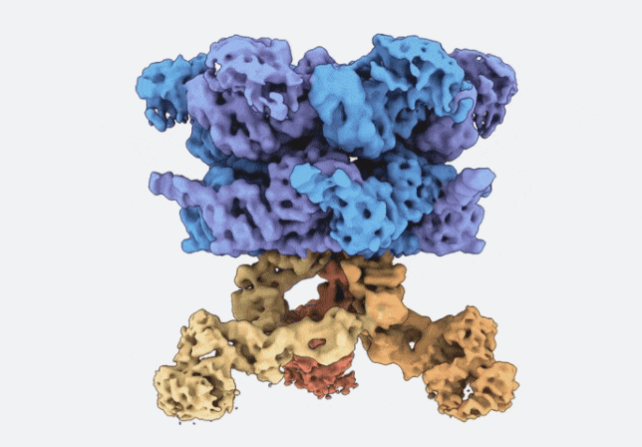
Three dimensional cryo-EM structure of VCP (blue and purple) bound to three VCPIP1 promoters (red, orange, and yellow). (Courtesy of Kapoor lab)
<関連情報>
- https://www.rockefeller.edu/news/37824-new-understanding-of-protein-processing-could-have-implications-for-alzheimers-and-some-cancers/
- https://rupress.org/jcb/article-abstract/224/5/e202410148/277322/Structural-insights-into-the-coupling-between-VCP
必須アンフォールダーゼであるVCPと脱ユビキチン化酵素の結合に関する構造的洞察
Structural insights into the coupling between VCP, an essential unfoldase, and a deubiquitinase
Lauren E. Vostal,Noa E. Dahan,Matthew J. Reynolds,Lily I. Kronenberg,Tarun M. Kapoor
Journal of Cell Biology Published:March 14 2025
DOI:https://doi.org/10.1083/jcb.202410148
Proteostasis involves degradation and recycling of proteins from organelles, membranes, and multiprotein complexes. These processes can depend on protein extraction and unfolding by the essential mechanoenzyme valosin-containing protein (VCP) and on ubiquitin chain remodeling by ubiquitin-specific proteases known as deubiquitinases (DUBs). How the activities of VCP and DUBs are coordinated is poorly understood. Here, we focus on the DUB VCPIP1, a VCP interactor required for post-mitotic Golgi and ER organization. We determine ∼3.3 Å cryogenic electron microscopy structures of VCP-VCPIP1 complexes in the absence of added nucleotide or the presence of an ATP analog. We find that up to 3 VCPIP1 protomers interact with the VCP hexamer to position VCPIP1’s catalytic domain at the exit of VCP’s central pore, poised to cleave ubiquitin following substrate unfolding. We observe competition between VCPIP1 and other cofactors for VCP binding and show that VCP stimulates VCPIP1’s DUB activity. Together, our data suggest how the two enzyme activities can be coordinated to regulate proteostasis.