2025-06-11 東京理科大学,産業技術総合研究所,新潟大学
<関連情報>
- https://www.tus.ac.jp/today/archive/20250602_9384.html
- https://onlinelibrary.wiley.com/doi/10.1002/pro.70147
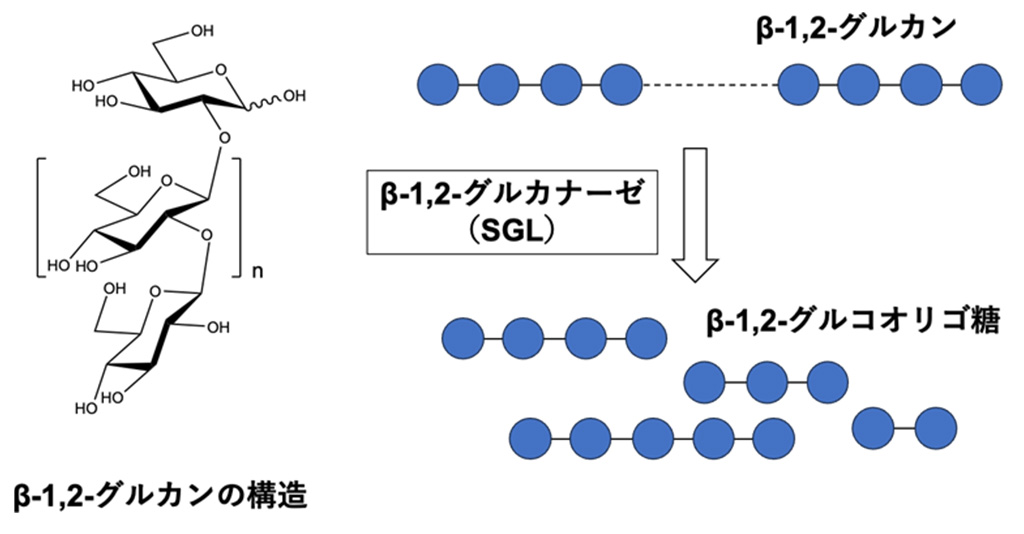
β-1,2-グルカナーゼの新しいグリコシド加水分解酵素ファミリー New glycoside hydrolase families of β-1,2-glucanases
Masahiro Nakajima, Nobukiyo Tanaka, Sei Motouchi, Kaito Kobayashi, Hisaka Shimizu, Koichi Abe, Naoya Hosoyamada, Naoya Abara, Naoko Morimoto, Narumi Hiramoto …
Protein Science Published: 24 May 2025
DOI:https://doi.org/10.1002/pro.70147
Abstract
β-1,2-Glucans are natural glucose polymers produced by bacteria and play important physiological roles, including as symbiotic or pathogenic factors and in osmoregulation. Glycoside hydrolase (GH) families related to β-1,2-glucan metabolism (GH144, GH162, and GH189) have recently been created by identification of two β-1,2-glucanases and a β-1,2-glucanotransferase, respectively. In this study, we further found four phylogenetically new groups with unknown functions (Groups 1–4) by sequence database analysis using enzymes from GH144 and GH162 as queries. Biochemical analysis of representative proteins in these groups revealed that the proteins in Groups 1–3 showed hydrolytic activity specific to β-1,2-glucan, while no substrate was found for the Group 4 protein. The kinetic parameters of the enzymes of Groups 1–3 were similar to GH144 and GH162 β-1,2-glucanases, indicating that these enzymes were β-1,2-glucanases. Optical rotation analysis revealed that the β-1,2-glucanases followed an anomer-inverting mechanism. Structural analysis of the proteins in Groups 1–4 revealed that they possess (α/α)6-barrel folds similar to those of GH144, GH162, and GH189 enzymes. Comparison of spatial positions of predicted acidic catalytic residues suggested that Groups 1–3 and GH144 had the same reaction mechanism. Overall, phylogenetic, biochemical, and structural analyses revealed that Groups 1–3 are new GH families, GH192, GH193, and GH194, respectively, and that the three families belong to clan GH-S (clan GH, classification based on structural similarity) as GH144 and GH162.