2025-06-16 ブラウン大学
ブラウン大学の研究者は、オピオイド使用障害の治療や新生児の薬物曝露評価に向けた微量血液検査法を開発。成人には指先からの血清で6種のオピオイドを迅速に自動測定、新生児には乾燥血液スポットを用い、初めて定量的に薬物曝露を評価可能とした。いずれも少量検体で高精度な分析が可能で、診断精度と治療支援の向上が期待される。研究成果は『Scientific Reports』『SLAS Technology』誌に掲載。
<関連情報>
- https://www.brown.edu/news/2025-06-16/opioids
- https://www.nature.com/articles/s41598-025-99130-5
- https://www.slas-technology.org/article/S2472-6303(25)00040-8/fulltext
ヒト実験室研究におけるオピオイド使用を評価するためのマイクロサンプリング技術によるオピオイド定量化 Opioid quantification via microsampling techniques to assess opioid use in human laboratory studies
Ramisa Fariha,Emma Rothkopf,Carolina L. Haass-Koffler & Anubhav Tripathi
Scientific Reports Published:21 May 2025
DOI:https://doi.org/10.1038/s41598-025-99130-5
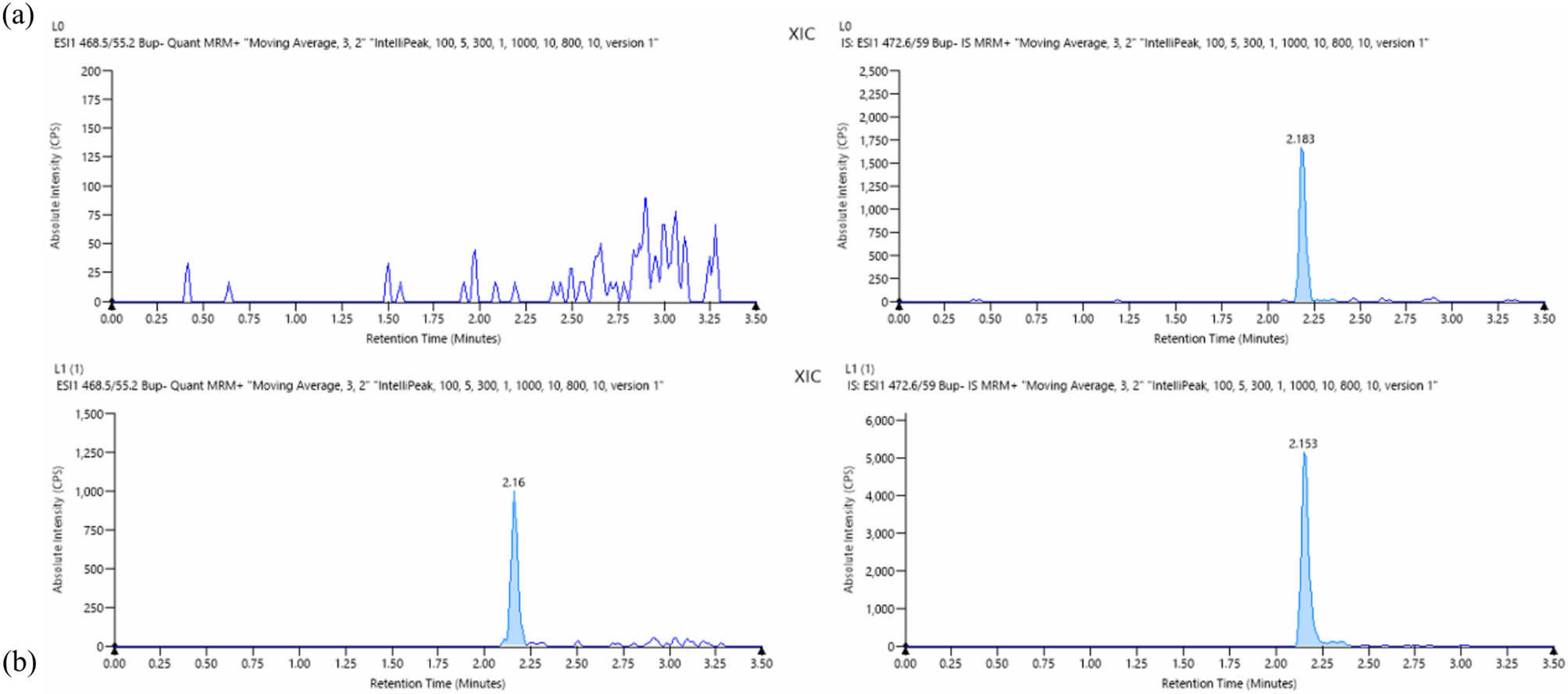
Abstract
Despite progress in neurobiological studies with human subjects, sample availability remains a challenge. Urine samples, widely used for screening, suffer from false-positive results due to immunoassay cross-reactivity. Serum, used for confirmatory testing, offers advantages but faces limitations due to blood collection. Microsamples, with a working volume less than 50 μL, present an ideal strategy for robust quantitative data collection in investigations and human laboratory studies. We developed, validated, and automated a serum-based LC-MS/MS assay for accurate quantification of six opioids using only 20 μL of patient samples. Our method, applied in a clinical trial with patients with opioid use disorder (N = 20) receiving intranasal oxytocin, or placebo, for one week in addition to opioid agonist therapy (buprenorphine or methadone). We quantified six different opioids, undetected by urine strip tests, that were used by patients during the treatment phase. Our high-throughput, automated approach surpasses existing methods in literature, enhancing efficiency in multi-matrix studies.
新生児禁忌症候群検出のための乾燥血液スポットの電場アシスト自動化可能なハイスループット試料調製による精度向上 Precision through electric-field assisted automatable high throughput sample preparation of dried blood spots for neonatal abstinence syndrome detection
Ramisa Fariha ∙ John Murphy ∙ Nondi Walters ∙ … ∙ Oluwanifemi D. Okoh ∙ Nabil M. Lawandy ∙ Anubhav Tripathi
SLAS Technology Published:March 26, 2025
DOI:https://doi.org/10.1016/j.slast.2025.100282
Abstract
In the United States, approximately 20 % of pregnant women disclose opioid misuse, contributing significantly to the widespread occurrence of Neonatal Abstinence Syndrome (NAS) in neonates exposed to opioids during gestation. Current NAS diagnosis heavily relies on clinical observation of symptoms, with the Finnegan Neonatal Abstinence Scoring System (FNASS) serving as the gold standard due to challenges associated with obtaining biological specimens from newborns. This methodological constraint poses difficulties in achieving accurate quantitative assessments and implementing timely therapeutic interventions. This study introduces a pioneering approach employing a cylindrical electrode-equipped device designed for the extraction of opioids from minute Dried Blood Spot (DBS) samples, thus optimizing the diagnostic pathway for NAS. The methodology integrates Liquid Chromatography tandem Mass Spectrometry (LC-MS/MS) for precise quantification of five distinct opioids. By demonstrating the efficacy of DBS microsamples as a robust quantitative diagnostic medium, this research highlights its potential to expedite NAS detection in infants. The innovative methodology promises superior diagnostic precision and accelerated processing times compared to current protocols, thereby addressing existing NAS diagnostic limitations and advancing maternal and infant healthcare practices.


