2025-06-25 滋賀医科大学
<関連情報>
- https://www.shiga-med.ac.jp/sites/default/files/2025-06/20250625_pr.pdf
- https://www.ahajournals.org/doi/10.1161/ATVBAHA.125.322619
アファジンはホスホリパーゼCと相互作用して血管平滑筋細胞の収縮を促進し、血圧調節のためのCa2+シグナルを増強する Afadin Promotes Vascular Smooth Muscle Cell Contraction by Interacting With Phospholipase C to Enhance Ca2+ Signaling for Blood Pressure Regulation
Md Mahbubur Rahman Khan, Akira Sato, Akio Shimizu, Shunji Suetaka, Md Rasel Molla, Masahiro Komeno, Mst Zenika Nasrin, Masanari Nishida, Futoshi Toyoda, Munehito Arai, and Hisakazu Ogita
Arteriosclerosis, Thrombosis, and Vascular Biology Published: 29 May 2025
DOI:https://doi.org/10.1161/ATVBAHA.125.322619
Abstract
BACKGROUND:
Vascular smooth muscle cells (VSMCs) regulate vascular tone and blood pressure. Stimulation of VSMCs with vasoconstrictors, such as AngII (angiotensin II) or norepinephrine, activates the G-protein–coupled receptor–mediated cascade, leading to a hypercontractile state and vascular remodeling. Afadin, an intracellular adaptor protein that mainly localizes at cell-cell junctions, regulates various biological phenomena. However, its role in VSMCs remains unclear.
METHODS:
VSMCs were isolated from newly generated VSMC-specific afadin conditional knockout mice. A small peptide (7 amino acids) designed in silico to inhibit the afadin–PLC (phospholipase C) β association was administered to the mouse VSMCs and aortic media using adeno-associated virus.
RESULTS:
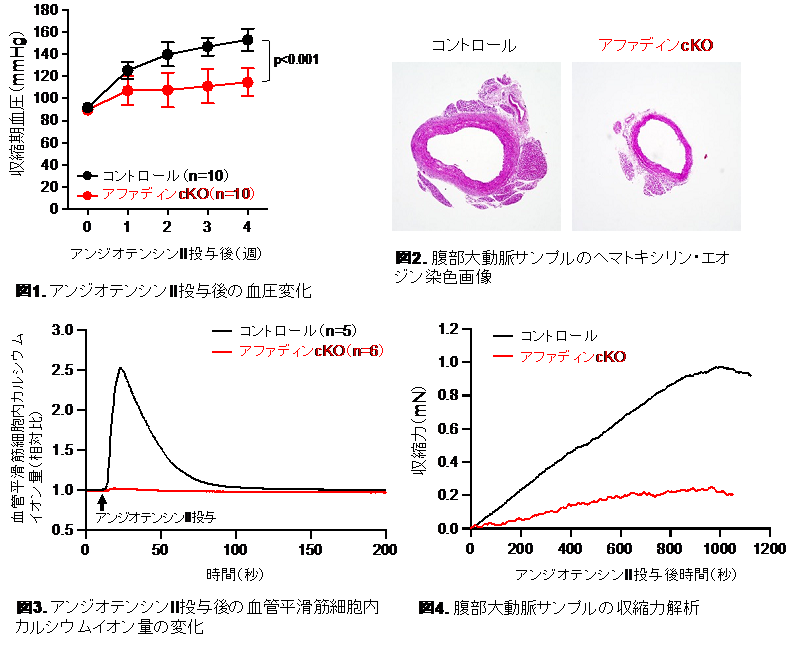
Unlike control mice, afadin conditional knockout mice did not exhibit AngII- or norepinephrine-induced elevation in blood pressure. VSMCs isolated from afadin conditional knockout mice were less responsive to AngII- or norepinephrine-induced cell contractility compared with control VSMCs, as evidenced by reduced release of intracellular Ca2+ resulting from lowered production of AngII- or norepinephrine-induced inositol 1,4,5-trisphosphate. Mechanistically, the PDZ domain of afadin was shown to associate with the C terminus of PLCβ, providing support for the localization of PLCβ on the plasma membrane, where it generates inositol 1,4,5-trisphosphate. Furthermore, the newly designed small peptide, which inhibited the afadin-PLCβ association, attenuated AngII-induced cell contractility and intracellular Ca2+ release in vitro and blocked AngII-stimulated blood pressure elevation in vivo.
CONCLUSIONS:
Afadin expression in VSMCs promotes cell contraction by interacting with PLCβ to enhance Ca2+ signaling and has potential as a novel molecular target for blood pressure regulation.