2025-07-11 京都大学 iPS細胞研究所
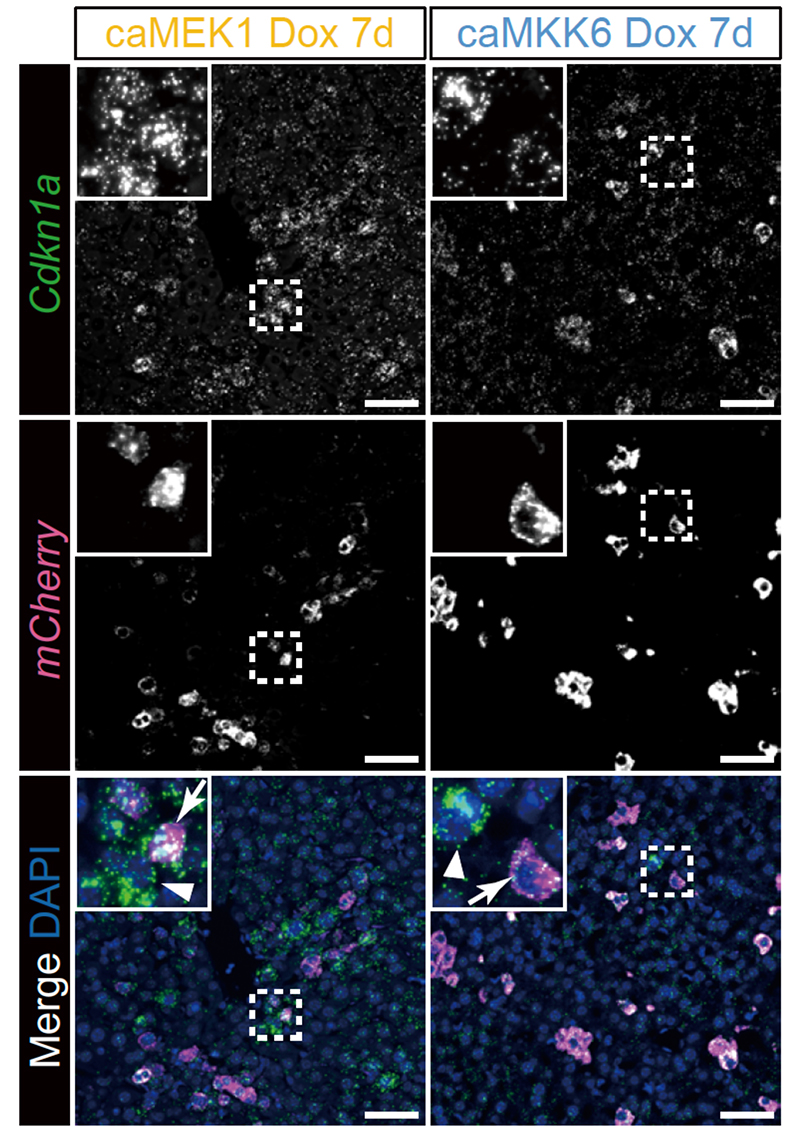
Fig.1 Dox処理後7日目の肝臓におけるRNA in situ解析
<関連情報>
- https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/250711-180000.html
- https://www.nature.com/articles/s43587-025-00917-y
生体内における一次老化と二次老化の特性解析 Characterizing primary and secondary senescence in vivo
Yuko Sogabe,Hirofumi Shibata,Mio Kabata,Akito Tanaka,Kanae Mitsunaga,Kazunori Sunadome,May Nakajima-Koyama,Michitada Hirano,Eisuke Nishida,Knut Woltjen,Hiroshi Seno,Yasuhiro Yamada & Takuya Yamamoto
Nature Aging Published:11 July 2025
DOI:https://doi.org/10.1038/s43587-025-00917-y
Abstract
There is robust evidence that senescence can be propagated in vitro through mechanisms including the senescence-associated secretory phenotype, resulting in the non-cell-autonomous induction of secondary senescence. However, the induction, regulation and physiological role of secondary senescence in vivo remain largely unclear. Here we generated senescence-inducible mouse models expressing either the constitutively active form of MEK1 or MKK6 and mCherry, to map primary and secondary senescent cells. Our models recapitulate characteristic features of senescence and demonstrate that primary and secondary phenotypes are highly tissue- and inducer-dependent. Spatially resolved RNA expression analyses at the single-cell level reveal that each senescence induction results in a unique transcriptional profile—even within cells of the same cell type—explaining the heterogeneity of senescent cells in vivo. Furthermore, we show that interleukin-1β, primarily derived from macrophages, induces secondary phenotypes. Our findings provide insight into secondary senescence in vivo and useful tools for understanding and manipulating senescence during aging.