2025-07-16 レンセラー工科大学(RPI)
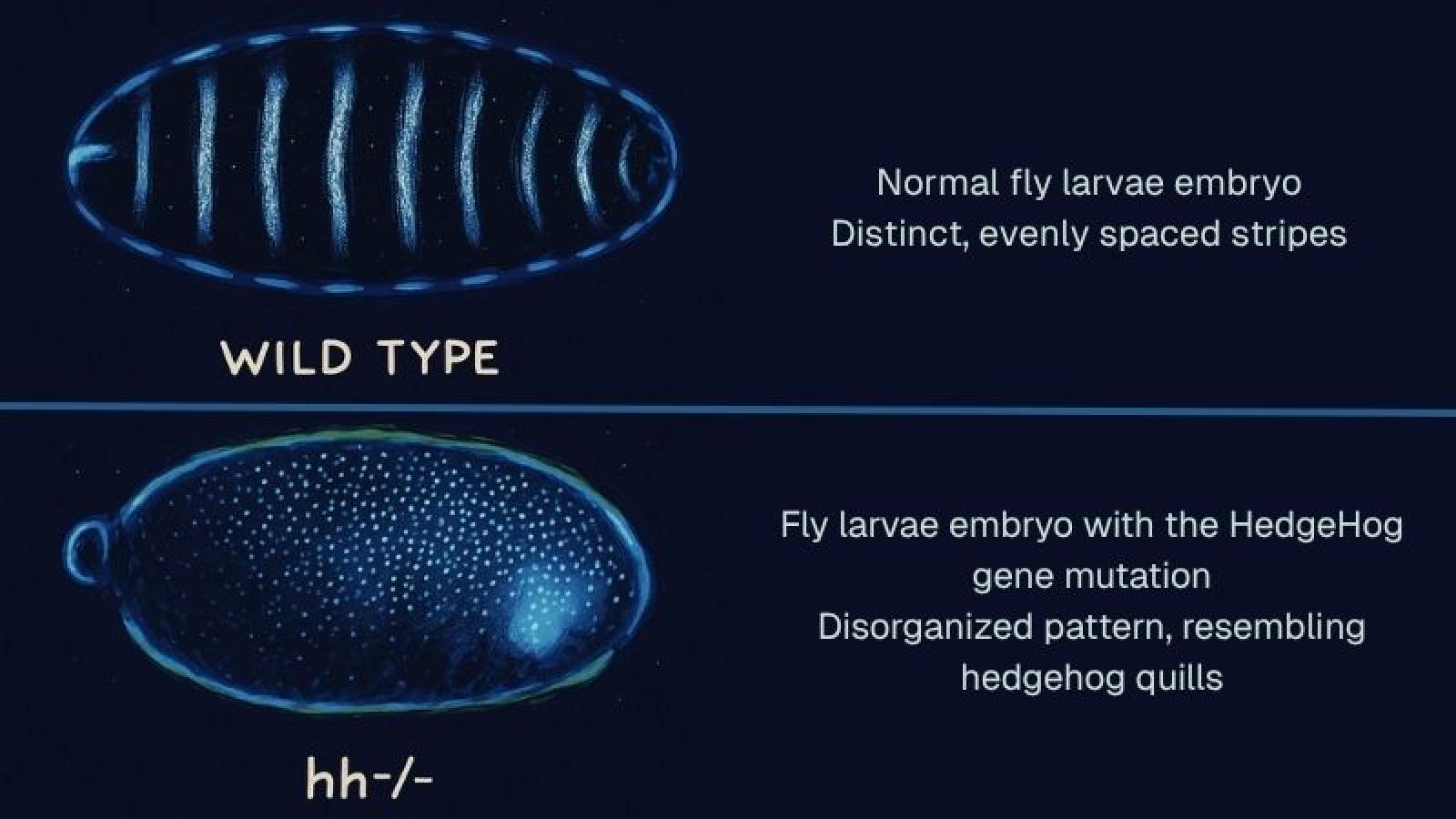 Two fruit fly embryos — one normal, one with the HedgeHog mutation — which demonstrate the crucial role of the gene in body segment patterning during development
Two fruit fly embryos — one normal, one with the HedgeHog mutation — which demonstrate the crucial role of the gene in body segment patterning during development
<関連情報>
- https://news.rpi.edu/2025/07/16/new-way-treat-cancer-targeting-hedgehog-proteins-hidden-weakness
- https://www.pnas.org/doi/10.1073/pnas.2415144122
保存されたC143は、ヘッジホッグの自己処理において分岐した中間体を形成する: ヘッジホッグシグナルに対するがん創薬ターゲット Conserved C143 forms a branched intermediate in Hedgehog autoprocessing: A cancer drug discovery target against Hedgehog signaling
Shannon Faris, Ke Xia, Andrew G. Wagner, +5 , and Chunyu Wang
Proceedings of the National Academy of Sciences Published:April 24, 2025
DOI:https://doi.org/10.1073/pnas.2415144122
Significance
Hedgehog (Hh) signaling plays fundamental roles in embryonic development while its abnormal activation in adults is associated with cancer. Hh-targeting drugs have gained FDA approval, but resistance has emerged quickly, underlining the need for novel types of Hh inhibitors. The catalytic mechanism uncovered here suggests that C143 may serve as a target for covalent inhibitors and drug design for novel Hh inhibitors.
Abstract
Hedgehog (Hh) signaling plays fundamental roles in embryonic development while its abnormal activation in adults is associated with cancer. Hh targeting drugs have gained FDA approval but resistance emerged quickly, underlining the need for novel types of Hh inhibitors. Hh signaling is initiated by the Hh ligand, generated from the autoprocessing of Hh precursor. However, the catalytic role of a highly conserved Hedgehog residue C143 is still poorly understood. Here, we confirmed that C143 is required for Hh autoprocessing in mammalian cells. NMR titration showed that C143 has an extremely low pKa of 4.5, befitting a highly reactive catalytic residue. We further established that Hh autoprocessing involves a branched intermediate (BI) with two N-termini, formed as a thioester on the C143 sidechain. BI migrates slower than the linear Hh precursor on SDS-PAGE and disappears with DTT treatment. With trypsin digestion and LC–MS/MS, we detected the N-terminal fragment from BI, which is absent from the linear Hh precursor. Therefore, C143 mediates the formation of a BI thioester in Hh autoprocessing, with a catalytic role equivalent to C + 1 in intein splicing. These findings bring us closer to a full mechanistic understanding of Hh autoprocessing while unifying the first two catalytic steps of Hh autoprocessing with intein splicing, its likely evolutionary predecessor. C143 can also serve as a target for covalent drugs for inhibiting Hh signaling in cancer.


