2025-08-12 カリフォルニア大学サンタバーバラ校 (UCSB)
<関連情報>
- https://news.ucsb.edu/2025/021987/new-method-synthesize-carbohydrates-could-pave-way-biomedical-advances
- https://www.nature.com/articles/s44160-025-00846-z
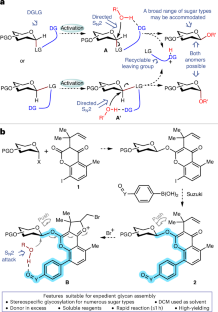
広く適用可能な立体特異的グリコシル化 A broadly applicable stereospecific glycosylation
Qing Zhang,Nils J. Flodén,Yongliang Zhang,Jielin Yang,Philip Kohnke,José Danglad-Flores,Eric T. Sletten,Peter H. Seeberger & Liming Zhang
Nature Synthesis Published:12 August 2025
DOI:https://doi.org/10.1038/s44160-025-00846-z
Abstract
The development of a general strategy for stereospecific construction of every type of glycosidic linkage remains a much sought-after yet unrealized goal. Such a strategy would be particularly useful in the context of complex glycan syntheses. Glycosylations involving an SN2 mechanism are ideal to ensure stereospecificity but have been challenging to implement in a manner conferring generality across a range of sugars. Here we disclose a stereospecific glycosylation method that accommodates a broad range of monosaccharides, including hexopyranoses (for example, glucose, galactose, mannose, fucose, alluronate, 2-azido-2-deoxyglucose and 2-azido-2-deoxygalactose) and pentofuranoses (for example, arabinose, ribose, xylose and lyxose). Mild activation with an electrophilic bromine reagent results in complete inversion of the anomeric configuration and excellent yields for many glycosylations. The method proved reliable in multistep oligosaccharide syntheses and automated glycan assembly.


