2025-08-21 オックスフォード大学
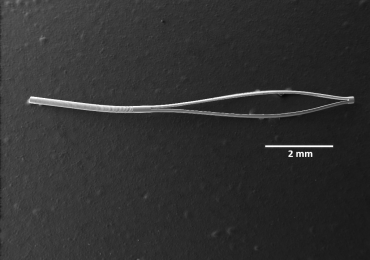
Scanning electronic microscopy image of the microstent. Credit: Yunlan, Zhang, Zhong You, Jared Ching.
<関連情報>
- https://www.ox.ac.uk/news/2025-08-21-oxford-researchers-develop-uniquely-shaped-microstent-combat-glaucoma
- https://www.cell.com/the-innovation/fulltext/S2666-6758(25)00138-9
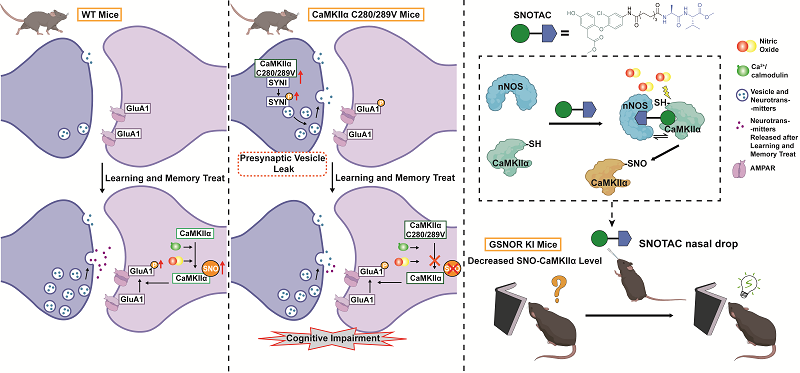
緑内障の治療のための新たな展開可能なマイクロステント A novel deployable microstent for the treatment of glaucoma
Yunlan Zhang ∙ Weijia Zhang ∙ Yunfang Yang ∙ … ∙ Chun Zhang ∙ Zhong You ∙ Jared Ching
The Innovation Published:April 29, 2025
DOI:https://doi.org/10.1016/j.xinn.2025.100935
Dear Editor,
Glaucoma is the second leading cause of blindness worldwide, after cataracts, and results in irreversible optic nerve damage and vision loss.1 The most common subtype, primary open-angle glaucoma (POAG), is caused by impaired aqueous humor (AH) drainage through the trabecular meshwork. POAG progresses gradually, often without symptoms until significant vision loss occurs. Treatments focus on lowering IOP using medicated eye drops, laser therapy, or surgical interventions. In the past decade, minimally invasive glaucoma surgery (MIGS) has emerged as a safer alternative to traditional surgeries, aiming to reduce IOP with minimal scleral or conjunctival disruption and a lower risk of fibrosis. MIGS devices improve AH drainage through the SCS, Schlemm’s canal, or the suprachoroidal space (Figure 1A). Among these approaches, tubular implants that drain from the anterior chamber (AC) to the SCS have demonstrated the most significant IOP reduction.2,3 Constructed from soft, biocompatible materials such as porcine gelatin and poly(styrene-block-isobutylene-block-styrene), these devices emulate traditional trabeculectomy surgery by forming a subconjunctival bleb, which is known for its potent IOP-lowering capabilities.4 However, clinical studies5 have revealed that the long-term effectiveness of these implants falls short of trabeculectomy due to issues like fibrosis and the eventual loss of bleb function, even with the use of anti-fibrosis medications.6 Additionally, these devices are susceptible to breakage and migration over time.

Figure 1 Design, fabrication, and experimental testing of the deployable microstent