2025-09-05 ノースウェスタン大学
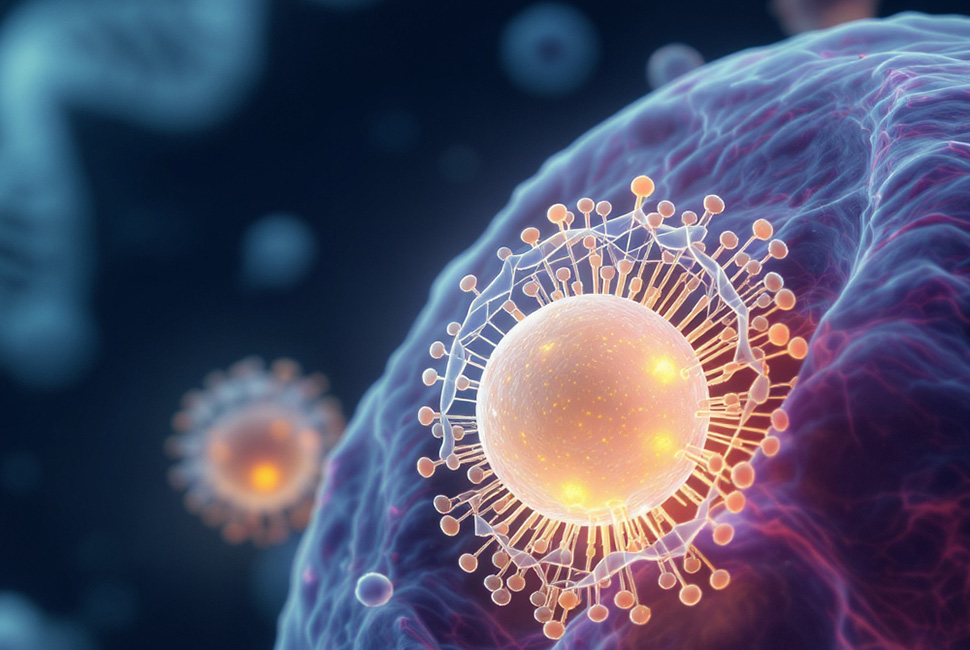
An artistic interpretation of a spherical nucleic acid (SNA) nanoparticle, carrying CRISPR cargo, entering a cell. When inside an SNA nanoparticle, CRISPR machinery enters cells three times more effectively. Image by the Mirkin Research Group
<関連情報>
- https://news.northwestern.edu/stories/2025/09/crisprs-efficiency-triples-with-dna-wrapped-nanoparticles/
- https://www.pnas.org/doi/10.1073/pnas.2426094122
CRISPR脂質ナノ粒子球状核酸を用いた汎用ゲノム編集戦略 A general genome editing strategy using CRISPR lipid nanoparticle spherical nucleic acids
Zhenyu Han, Chi Huang, Taokun Luo, and Chad A. Mirkin
Proceedings of the National Academy of Sciences Published:September 4, 2025
DOI:https://doi.org/10.1073/pnas.2426094122
Significance
Genome editing holds broad therapeutic potential, but clinical translation requires efficient delivery of genome editors to target cells and tissues. We modified lipid nanoparticles (LNPs) with a dense shell of DNA to create CRISPR LNP–spherical nucleic acids (LNP–SNAs), which exhibit enhanced cellular uptake, biocompatibility, and transfection efficiency. LNP–SNAs enable both insertion–deletion (indel) formation, which disrupts gene function, and homology-directed repair (HDR) for precise, user-defined genome modifications. Across multiple cell lines and genomic targets, LNP–SNAs achieve indel rates of 15 to 68% and HDR efficiencies of 13 to 30%, representing a 1.5 to 3-fold improvement over LNPs. These findings establish CRISPR LNP–SNAs as an effective platform for genome editing.
Abstract
Genome editing with CRISPR–Cas systems hold promise for treating a wide range of genetic disorders and cancers. However, efficient delivery of genome editors remains challenging due to the requirement for the simultaneous delivery or intracellular generation of Cas proteins, guide RNAs, and, in some applications, donor DNAs. Furthermore, the immunogenicity and toxicity of delivery vehicles can limit the safety and efficacy of genetic medicines. Here, we combine two nucleic acid delivery approaches to create CRISPR lipid nanoparticle–spherical nucleic acids (LNP–SNAs) that are both efficient and biocompatible. Compared to lipid nanoparticles (LNPs) lacking a surface-bound DNA shell, CRISPR LNP–SNAs exhibit two- to three-fold higher cellular uptake, reduced cytotoxicity, and improved gene transfection efficiency. Across multiple cell lines and genomic loci, CRISPR LNP–SNAs induce insertion–deletion mutations at average frequencies two- to three-fold higher than those observed with LNPs. When codelivered with donor templates, CRISPR LNP–SNAs enable homology-directed repair at an average efficiency of 21 ± 7%, a 2.5-fold improvement over LNPs (8 ± 4%). The ease of synthesis and biocompatibility of CRISPR LNP–SNAs highlight their potential as a versatile delivery platform for CRISPR–Cas and other gene therapies.