2025-09-12 中国科学院(CAS)
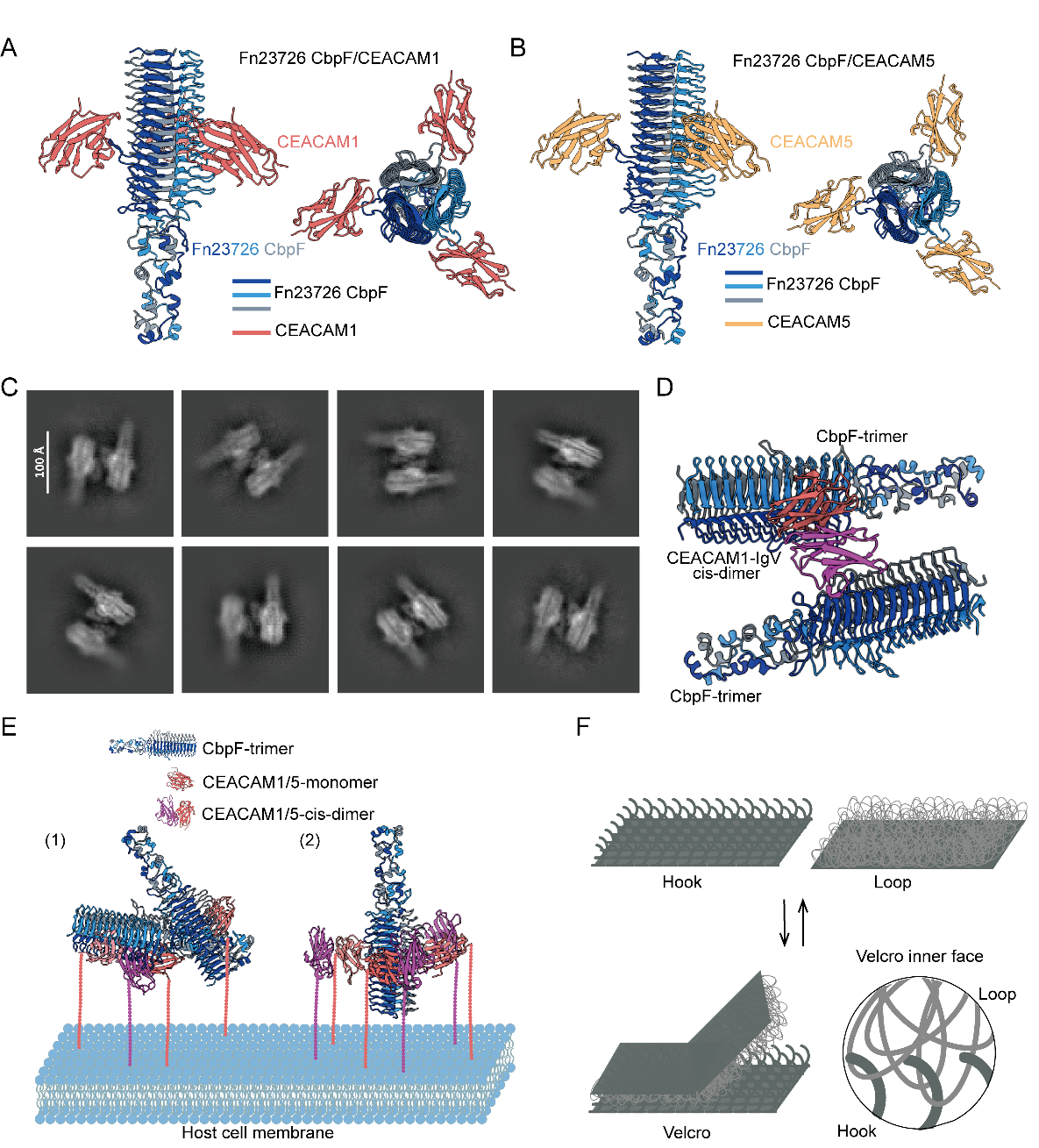
Structural model of CbpF binding to CEACAM1/CEACAM5 and the proposed “Velcro” adhesion mechanism. (Image by Prof. George F. Gao’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202509/t20250917_1054973.shtml
- https://www.pnas.org/doi/10.1073/pnas.2516574122
フソバクテリウム・ヌクレアタム自己輸送体接着因子CbpFの人間CEACAM1およびCEACAM5への結合:細菌付着のベルクロモデル Binding of Fusobacterium nucleatum autotransporter adhesin CbpF to human CEACAM1 and CEACAM5: A Velcro model for bacterium adhesion
Fan Shen, Linjie Li, Dongchun Yang, +8 , and George F. Gao
Proceedings of the National Academy of Sciences Published:September 10, 2025
DOI:https://doi.org/10.1073/pnas.2516574122
Significance
Fusobacterium nucleatum is associated with the initiation, metastasis, and immune evasion of colorectal cancer (CRC). Its adhesin CbpF promotes CRC progression by binding to host receptors CEACAM1 and CEACAM5. In this study, we resolve the structures of CbpF in complex with either human CEACAM1 or CEACAM5. We identify the Q78 residue in both receptors as molecular determinant for CbpF’s selective binding. Furthermore, we propose a Velcro-like bacterium–host adhesion model, wherein flexible bacterial proteins interdigitate with host receptor through multiple binding sites to stabilize interactions. This work provides a molecular-level explanation for plasticity of bacterial adhesion, deepening our understanding of pathogen–host interactions and laying foundation for developing anti-tumor colonization therapies. Notably, the Velcro model may fit other flexible bacterum–host adhesion interactions.
Abstract
In eukaryotic systems, three major types of cell junctions have been well characterized. While bacterial adhesion mechanisms also exhibit remarkable diversity, the molecular processes that regulate the dynamic modulation of binding strength between elongated bacterial cells and host cells remain poorly understood. Fusobacterium nucleatum (F. nucleatum) utilizes the surface adhesin CbpF to interact with the highly expressed host receptors CEACAM1 and CEACAM5 on cancer cells to facilitate tumor colonization. By elucidating the structural details of CbpF binding to human CEACAM1/CEACAM5 receptors, and through mechanistic investigations, we identified that the prominent EFNGQYQ loop on CbpF and the key Q78 residue of CEACAM1/CEACAM5 constitute the molecular linchpin of this pathogen–host interface. Furthermore, we found a distinct type of binding particle and proposed a Velcro-like adhesion model. In this model, CbpF mediates robust attachment through the simultaneous interaction of multiple binding sites, akin to the interlocking mechanism of Velcro. This multivalent interaction allows F. nucleatum to dynamically switch between firm anchoring and easy detachment, adapting to varying physiological microenvironments. Our study elucidates the dynamic modulation of bacterial adhesion strength and lays the foundation for developing therapeutic interventions to disrupt the bacterium–host interface.