2025-09-08 浙江大学(ZJU)
<関連情報>
- https://www.zju.edu.cn/english/2025/0908/c19573a3078724/page.psp
- https://jhoonline.biomedcentral.com/articles/10.1186/s13045-025-01721-2
BRD4はRhoBの転写抑制因子として作用し、末梢赤血球生成を阻害する BRD4 acts as a transcriptional repressor of RhoB to inhibit terminal erythropoiesis
Yijin Chen,Dawei Huo,Ye Meng,Jie Zhang,Mengmeng Huang,Qian Luo,Yulin Xu,Haiqiong Zheng,Yingli Han,Xiangjun Zeng,Yanjuan Liu,Yunfei Liu,Rui Wen,Delin Kong,Ruxiu Tie,Shanshan Pei,Nan Liu,Pengxu Qian,He Huang & Meng Zhang
Journal of Hematology & Oncology Published:01 July 2025
DOI:https://doi.org/10.1186/s13045-025-01721-2
Abstract
Background
Terminal erythropoiesis is a complex multistep process involving coordination of gene transcription and dramatic nuclear condensation, which leads to the expulsion of nuclei to generate reticulocytes. However, we lack a comprehensive understanding of the key transcriptional and epigenetic regulators involved.
Methods
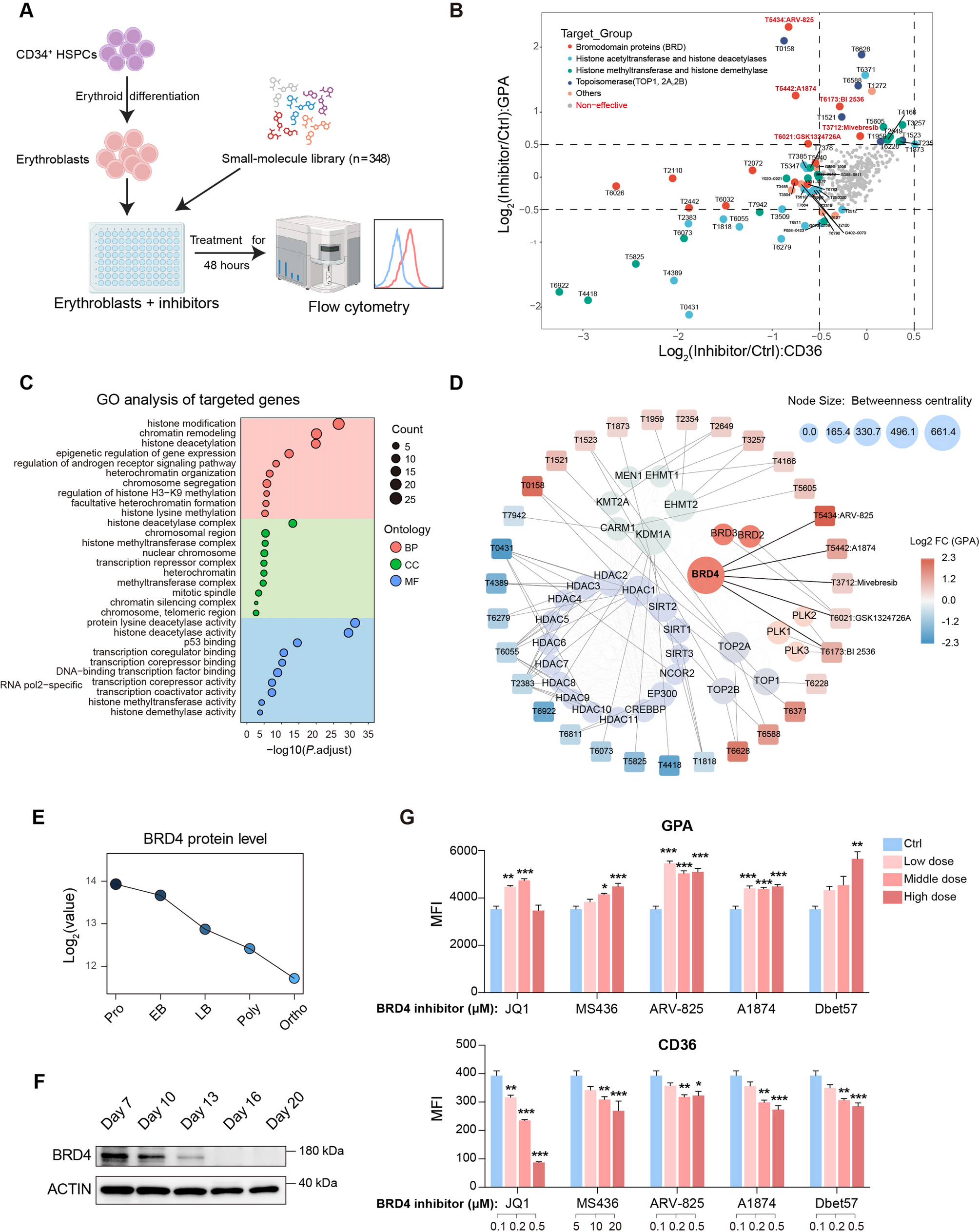
We used a high-throughput small molecule screen in primary CD34+-derived human erythroblasts to identify targets that promoted terminal erythropoiesis, and further confirmed the phenotype in different differentiation systems by inhibitors and shRNAs of different BRD4 isoforms. Then we performed RNA-seq, ATAC-seq, ChIP-qPCR, Co-IP, and reanalyzed previously-published transcriptional data and mass spectrometric data to clarify how BRD4 regulates terminal erythropoiesis.
Results
We identified that inhibitors of the bromodomain protein BRD4, an epigenetic reader and transcriptional activator together with CDK9, promoted terminal erythropoiesis from hematopoietic stem/progenitor cells and embryonic stem cells, and enhanced enucleation. Combined analysis of our RNA-seq, ATAC-seq, and previously-published transcriptional data of erythroblast differentiation at different stages confirmed that BRD4 inhibition accelerates erythroblast maturation. Unexpectedly, this BRD4 function was independent of its classical CDK9 interaction and transcriptional activation. Instead, RNA-seq, ATAC-seq, and Cut&Tag upon BRD4 inhibition revealed that BRD4 regulates erythropoiesis by inhibiting the small G protein RhoB and disrupts actin reorganization. ChIP-qPCR, Co-IP, and functional studies revealed that BRD4 acts as a transcriptional repressor by interacting with the histone methyltransferase EHMT1/2.
Conclusions
We demonstrate a non-classical role for BRD4 as a transcriptional repressor of RhoB to regulate erythroid maturation, and classical CDK9 dependent role to regulate cell proliferation of erythroblasts. Besides, we clarify RhoB’s activity and function during terminal erythropoiesis. BRD4 inhibition might be a simple method to promote in vitro blood cell production, and a candidate therapeutic target for diseases leading to dyserythropoiesis such as myelodysplastic syndromes.


