2025-09-22 基礎生物学研究所,総合研究大学院大学,東京大学定量生命科学研究所
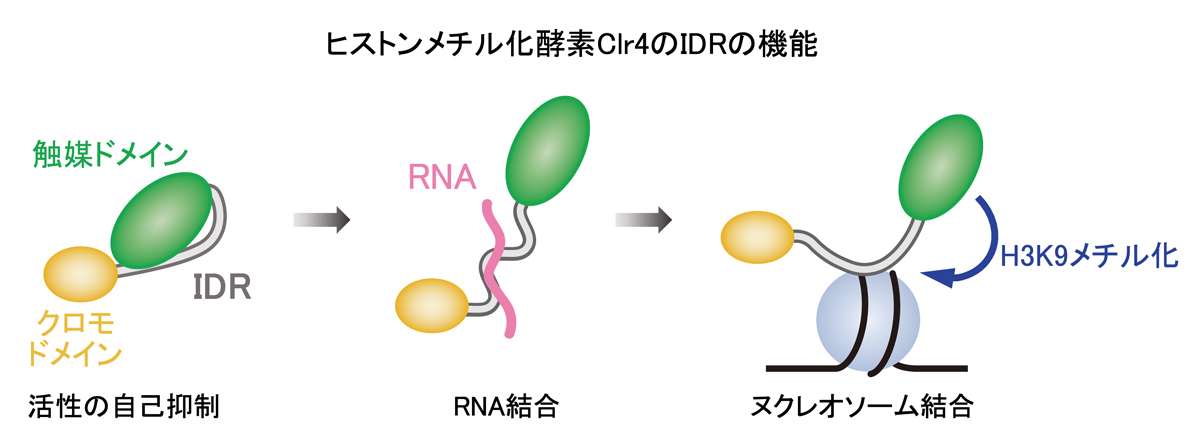
図1:ヒストンのメチル化酵素であるClr4内の天然変性領域(IDR)のアミノ酸は触媒ドメインの酵素活性を自己抑制する。IDRがRNAに結合するとその抑制は解除され、脱抑制が起こる。IDRはヌクレオソームに結合し、Clr4によるヌクレオソーム上のH3K9のメチル化を促進する。
<関連情報>
- https://www.nibb.ac.jp/press/2025/09/22.html
- https://academic.oup.com/nar/article/53/17/gkaf878/8249847?login=false
Clr4/Suv39の内在性無秩序領域は、その酵素活性を調節し、ヘテロクロマチン拡散を保証する Intrinsically disordered region of Clr4/Suv39 regulates its enzymatic activity and ensures heterochromatin spreading
Rinko Nakamura, Aki Hayashi, Reiko Nakagawa, Yuriko Yoshimura, Naoki Horikoshi, Hitoshi Kurumizaka, Jun-ichi Nakayama
Nucleic Acids Research Published:09 September 2025
DOI:https://doi.org/10.1093/nar/gkaf878
Abstract
Methylation of histone H3 at lysine 9 (H3K9me), a hallmark of heterochromatin, is catalyzed by Clr4/Suv39. Clr4/Suv39 contains two conserved domains—an N-terminal chromodomain and a C-terminal catalytic domain—connected by an intrinsically disordered region (IDR). Several mechanisms have been proposed to regulate Clr4/Suv39 activity, but how it is regulated under physiological conditions remains largely unknown. We found that the N-terminus of Clr4 interacts with its C-terminal catalytic domain and represses its enzymatic activity. Detailed biochemical analyses revealed that basic amino acid residues in the IDR are involved in this interaction. Amino acid substitutions of these residues weakened this interaction, thereby promoting Clr4 activity in vitro. Interestingly, cells expressing mutant Clr4 with these substitutions showed a silencing defect, which suggested additional roles of the IDR in vivo. Genetic analysis revealed that the IDR functions in H3K9me spreading and that this activity is functionally linked to the RNAi pathway. We also showed that Clr4 binds to RNAs via the IDR and that RNA attenuates Clr4 autoinhibition in vitro. Furthermore, the IDR was found to contribute to the targeting of nucleosomal substrates in vitro. These results reveal a novel function of the Clr4/Suv39 IDR in regulating its enzymatic activity and heterochromatin spreading.