2025-09-29 東京科学大学
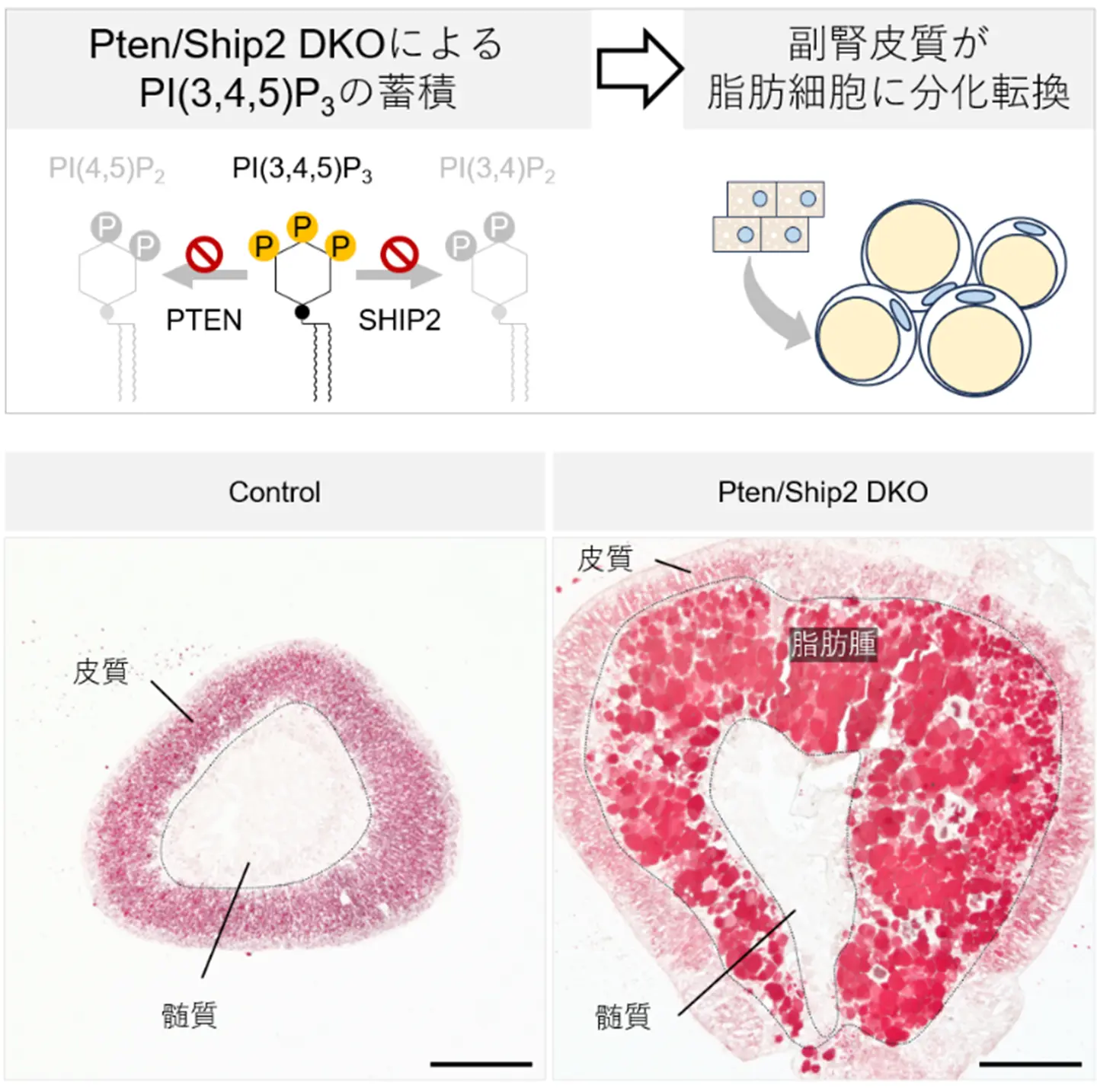 図1. (上) PI(3,4,5)P3の分解を担う二つの酵素Pten/Ship2を副腎皮質において欠損させたところ、副腎皮質が脂肪細胞に分化転換することを見出した。
図1. (上) PI(3,4,5)P3の分解を担う二つの酵素Pten/Ship2を副腎皮質において欠損させたところ、副腎皮質が脂肪細胞に分化転換することを見出した。
(下)副腎は皮質と髄質から構成される組織であるが、 Pten/Ship2を欠損した副腎では大量の脂肪細胞(脂肪腫)が皮質と髄質の境界部に出現した。
<関連情報>
PI(3,4,5)P 3 /AKT依存性副腎皮質細胞の脂肪細胞への分化転換を介した副腎脂肪腫の形成 Adrenal lipoma formation via PI(3,4,5)P3/AKT-dependent transdifferentiation of adrenocortical cells into adipocytes
Shogo Yanai, Junko Sasaki, Hyeon-Cheol Lee-Okada, +12 , and Takehiko Sasaki
Proceedings of the National Academy of Sciences Published:September 9, 2025
DOI:https://doi.org/10.1073/pnas.2510306122
Significance
Transdifferentiation—the conversion of one differentiated cell type into another—can be achieved experimentally through the introduction of exogenous transcription factors. Such experimental approaches have facilitated the elucidation of mechanisms underlying transdifferentiation, particularly the pivotal roles of specific transcription factors. Here, we demonstrate that hyperaccumulation of a single endogenous signaling lipid, PI(3,4,5)P3 is sufficient to drive in vivo transdifferentiation of adrenocortical cells into adipocyte-like cells in mice. This finding reveals a role for lipid-mediated signaling in regulating cell fate plasticity and establishes a nongenetic mechanism of cellular reprogramming. Furthermore, our study provides insights into the potential origin of adrenal lipomas and may serve as a foundation for developing therapeutic strategies that leverage endogenous signaling pathways to control cell identity.
Abstract
Adrenal lipomas are benign tumors containing ectopic adipose tissue in the adrenal gland, an organ that normally lacks both adipocytes and their progenitors. The origin of this ectopic fat remains enigmatic, and the absence of a genetic animal model has hindered its investigation. Phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], a key signaling lipid that regulates cellular growth and differentiation, is tightly regulated by the lipid phosphatases PTEN (phosphatase and tensin homolog) and SHIP2 (SH2-containing inositol phosphatase 2). Here, we demonstrate that simultaneous loss of Pten and Ship2 in the adrenal cortex induces adrenal lipoma formation in mice. These lipomatous cells display both adipocyte-like morphology and adipocyte-specific gene expression. Lineage tracing revealed that these lipomas originate from the adrenal cortex. Mechanistically, PI(3,4,5)P3 hyperaccumulation in the adrenal cortex activates AKT (AKT8 virus oncogene cellular homolog), leading to ectopic PPARγ (peroxisome proliferator activated receptor gamma) expression, a key driver of adipocyte differentiation. This study suggests that the PI(3,4,5)P3/AKT-driven transdifferentiation of adrenocortical cells may represent a central mechanism underlying adrenal lipoma formation, thereby providing insights into lipoma pathogenesis and cellular reprogramming in vivo.

