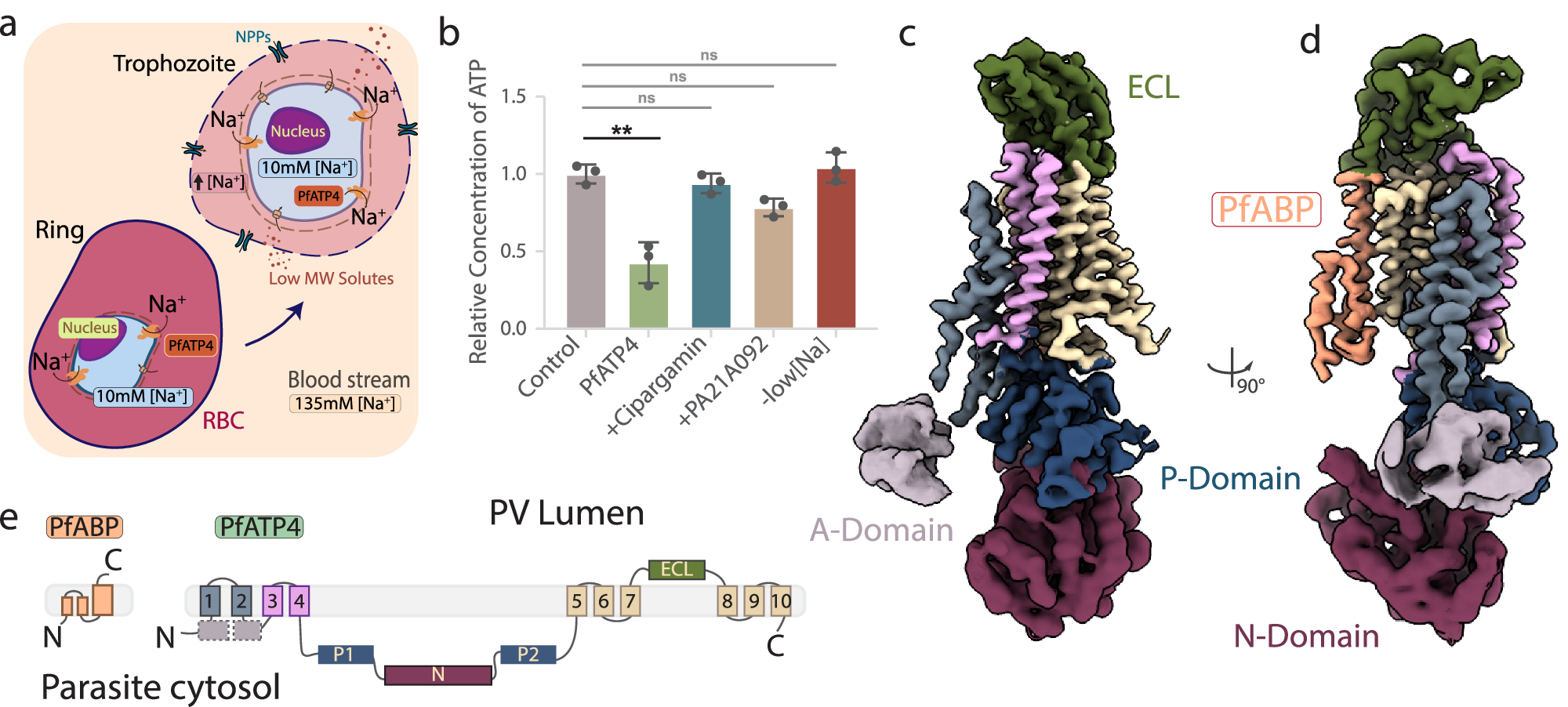
2025-10-21 カリフォルニア大学ロサンゼルス校(UCLA)
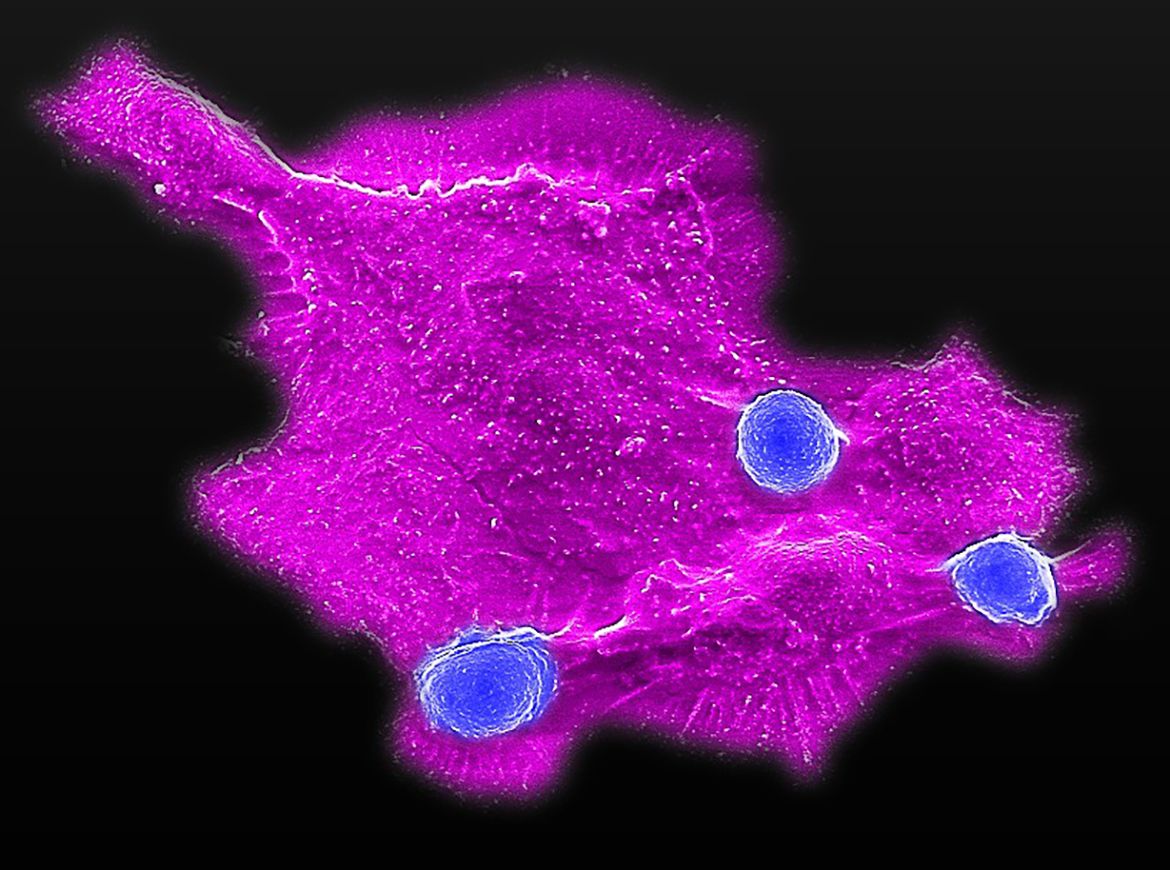
Lili Yang Lab/UCLA
Microscopy image showing blood stem cell-engineered CAR-NKT cells (blue) attacking a human solid tumor cell (magenta).
<関連情報>
- https://newsroom.ucla.edu/releases/ucla-scientists-develop-one-product-fits-all-immunotherapy-breast-cancer
- https://jhoonline.biomedcentral.com/articles/10.1186/s13045-025-01736-9
強力な抗腫瘍活性を有する臍帯血CD34⁺ HSPC由来メソテリン特異的CAR-NKT細胞を用いたトリプルネガティブ乳がんの標的化 Targeting triple-negative breast cancer using cord-blood CD34⁺ HSPC-derived mesothelin-specific CAR-NKT cells with potent antitumor activity
Yan-Ruide Li,Xinyuan Shen,Yichen Zhu,Zhe Li,Ryan Hon,Yanxin Tian,Jie Huang,Annabel S. Zhao,Nathan Y. Ma,Catherine Zhang,David Lin,Karine Sargsyan,Yuan Yuan & Lili Yang
Journal of Hematology & Oncology Published:13 October 2025
DOI:https://doi.org/10.1186/s13045-025-01736-9
Abstract
Background
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer characterized by the lack of ER, PR, and HER2 expression. Its aggressive behavior, high degree of tumor heterogeneity, and immunosuppressive tumor microenvironment (TME) are associated with poor clinical outcomes, rapid disease progression, and limited therapeutic options. Although chimeric antigen receptor (CAR)-engineered T cell therapy has shown certain promise, its applicability in TNBC is hindered by antigen escape, TME-mediated suppression, and the logistical constraints of autologous cell production.
Methods
In this study, we employed hematopoietic stem and progenitor cell (HSPC) gene engineering and a feeder-free HSPC differentiation culture to generate allogeneic IL-15-enhanced, mesothelin-specific CAR-engineered invariant natural killer T (Allo15MCAR-NKT) cells.
Results
These cells demonstrated robust and multifaceted antitumor activity against TNBC, mediated by CAR- and NK receptor-dependent cytotoxicity, as well as selective targeting of CD1d+ TME immunosuppressive cells through their TCR. In both orthotopic and metastatic TNBC xenograft models, Allo15MCAR-NKT cells demonstrated potent antitumor activity, associated with robust effector and cytotoxic phenotypes, low exhaustion, and a favorable safety profile without inducing graft-versus-host disease.
Conclusions
Together, these results support Allo15MCAR-NKT cells as a next-generation, off-the-shelf immunotherapy with strong therapeutic potential for TNBC, particularly in the context of metastasis, immune evasion, and treatment resistance.