コロンビアで神経変性疾患の原因となる希少な変異の状況を調査 Researchers survey the landscape of rare mutations behind neurodegenerative diseases in Colombia
2022-06-14 カリフォルニア大学サンタバーバラ校(UCSB)
その結果、数千年前にさかのぼり、いくつかの大陸にまたがる遺伝子の結果が得られたのである。この研究成果は、学術誌『Genome Medicine』に掲載されています。
<関連情報>
- https://www.news.ucsb.edu/2022/020658/confluence-rare-mutations
- https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-022-01035-9
創始者効果によるコロンビアの希少な突然変異がもたらす神経変性疾患景観 A neurodegenerative disease landscape of rare mutations in Colombia due to founder effects
Juliana Acosta-Uribe,David Aguillón,J. Nicholas Cochran,Margarita Giraldo,Lucía Madrigal,Bradley W. Killingsworth,Rijul Singhal,Sarah Labib,Diana Alzate,Lina Velilla,Sonia Moreno,Gloria P. García,Amanda Saldarriaga,Francisco Piedrahita,Liliana Hincapié,Hugo E. López,Nithesh Perumal,Leonilde Morelo,Dionis Vallejo,Juan Marcos Solano,Eric M. Reiman,Ezequiel I. Surace,Tatiana Itzcovich,Ricardo Allegri,Raquel Sánchez-Valle,Andrés Villegas-Lanau,Charles L. White III,Diana Matallana,Richard M. Myers,Sharon R. Browning,Francisco Lopera & Kenneth S. Kosik
Genome Medicine Published:08 March 2022
DOI:https://doi.org/10.1186/s13073-022-01035-9
Abstract
Background
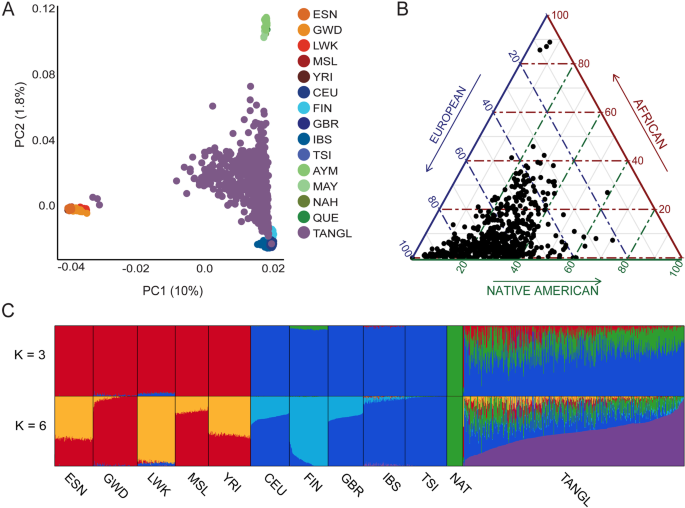
The Colombian population, as well as those in other Latin American regions, arose from a recent tri-continental admixture among Native Americans, Spanish invaders, and enslaved Africans, all of whom passed through a population bottleneck due to widespread infectious diseases that left small isolated local settlements. As a result, the current population reflects multiple founder effects derived from diverse ancestries.
Methods
We characterized the role of admixture and founder effects on the origination of the mutational landscape that led to neurodegenerative disorders under these historical circumstances. Genomes from 900 Colombian individuals with Alzheimer’s disease (AD) [n = 376], frontotemporal lobar degeneration-motor neuron disease continuum (FTLD-MND) [n = 197], early-onset dementia not otherwise specified (EOD) [n = 73], and healthy participants [n = 254] were analyzed. We examined their global and local ancestry proportions and screened this cohort for deleterious variants in disease-causing and risk-conferring genes.
Results
We identified 21 pathogenic variants in AD-FTLD related genes, and PSEN1 harbored the majority (11 pathogenic variants). Variants were identified from all three continental ancestries. TREM2 heterozygous and homozygous variants were the most common among AD risk genes (102 carriers), a point of interest because the disease risk conferred by these variants differed according to ancestry. Several gene variants that have a known association with MND in European populations had FTLD phenotypes on a Native American haplotype. Consistent with founder effects, identity by descent among carriers of the same variant was frequent.
Conclusions
Colombian demography with multiple mini-bottlenecks probably enhanced the detection of founder events and left a proportionally higher frequency of rare variants derived from the ancestral populations. These findings demonstrate the role of genomically defined ancestry in phenotypic disease expression, a phenotypic range of different rare mutations in the same gene, and further emphasize the importance of inclusiveness in genetic studies.