2025-10-28 エディンバラ大学
<関連情報>
- https://www.ed.ac.uk/news/oxygen-loss-raises-disease-risk-by-altering-genes
- https://www.nature.com/articles/s41590-025-02301-9
低酸素症は好中球前駆細胞におけるヒストンクリッピングとH3K4me3の喪失を誘発し、好中球免疫の長期的な障害をもたらす Hypoxia induces histone clipping and H3K4me3 loss in neutrophil progenitors resulting in long-term impairment of neutrophil immunity
Manuel A. Sanchez-Garcia,Pranvera Sadiku,Brian M. Ortmann,Niek Wit,Yutaka Negishi,Patricia Coelho,Ailiang Zhang,Chinmayi Pednekar,Andrew J. M. Howden,David M. Griffith,Rachel Seear,Jessica D. Kindrick,Janine Mengede,George Cooper,Tyler Morrison,Emily R. Watts,Benjamin T. Shimeld,Leila Reyes,Ananda S. Mirchandani,Simone Arienti,Xiang Xu,Alexander Thomson,Alejandro J. Brenes,Helena A. Turton,the PHOSP-COVID Study Collaborative Group,… Sarah R. Walmsley
Nature Immunology Published:28 October 2025
DOI:https://doi.org/10.1038/s41590-025-02301-9
Abstract
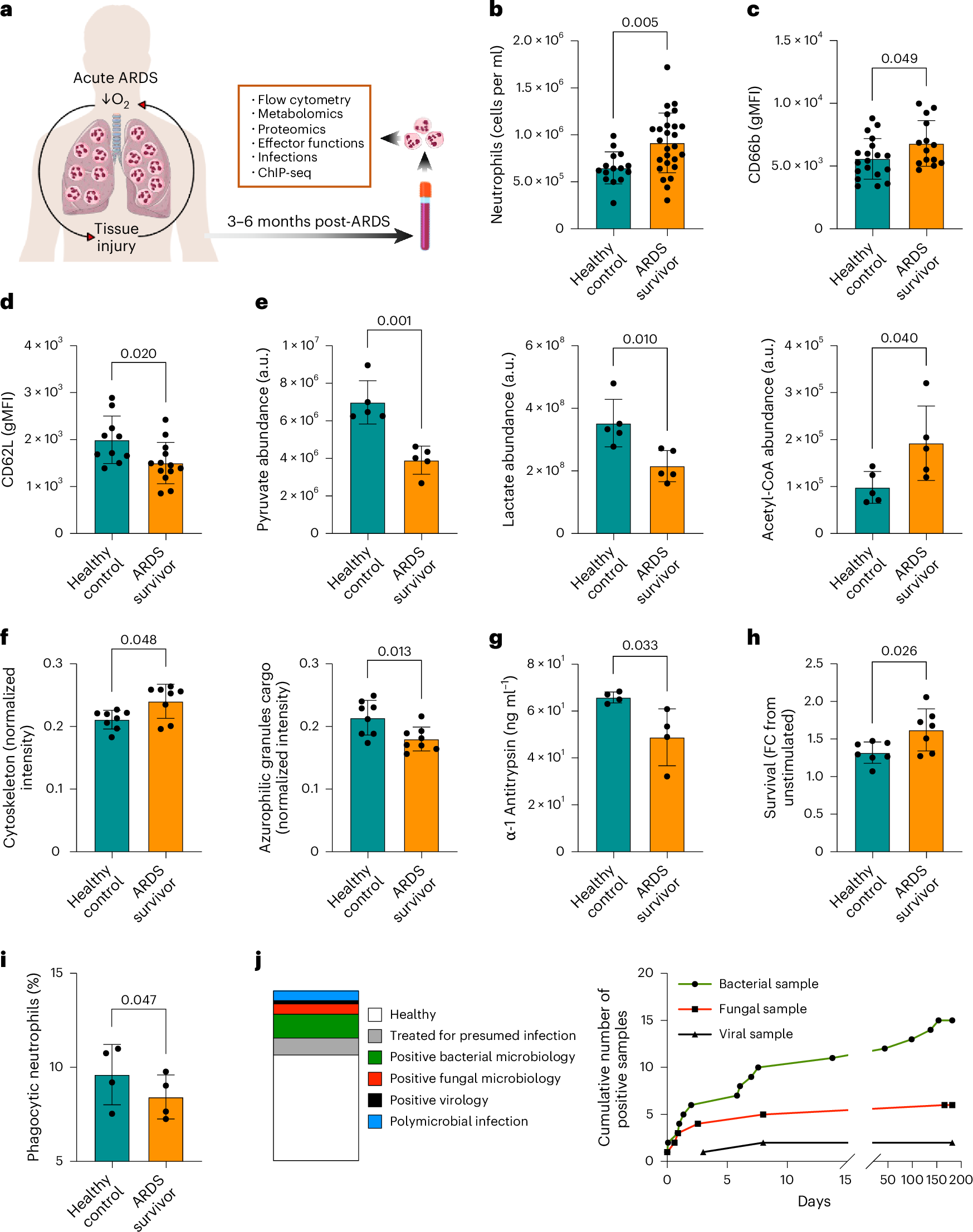
The long-term impact of systemic hypoxia resulting from acute respiratory distress syndrome (ARDS) on the function of short-lived innate immune cells is unclear. We show that patients 3–6 months after recovering from ARDS have persistently impaired circulating neutrophil effector functions and an increased susceptibility to secondary infections. These defects are linked to a widespread loss of the activating histone mark H3K4me3 in genes that are crucial for neutrophil activities. By studying healthy volunteers exposed to altitude-induced hypoxemia, we demonstrate that oxygen deprivation alone causes this long-term neutrophil reprogramming. Mechanistically, mouse models of systemic hypoxia reveal that persistent loss of H3K4me3 originates in proNeu and preNeu progenitors within the bone marrow and is linked to N-terminal histone 3 clipping, which removes the lysine residue for methylation. Thus, we present new evidence that systemic hypoxia initiates a sustained maladaptive reprogramming of neutrophil immunity by triggering histone 3 clipping and H3K4me3 loss in neutrophil progenitors.