2025-11-07 オックスフォード大学
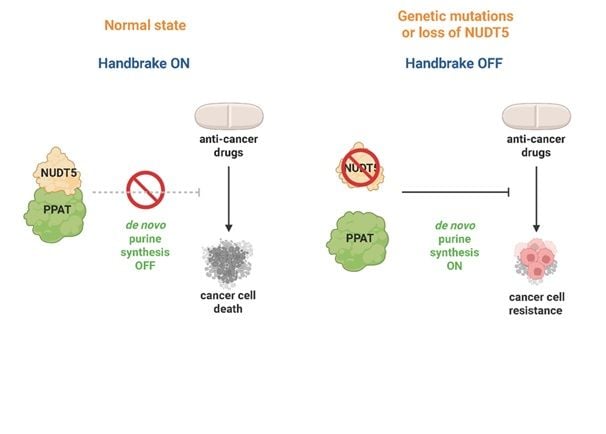
Releasing the Brake on Purine Metabolism. NUDT5 binds and restrains PPAT, acting as a cellular handbrake to limit the production of DNA building blocks. Loss or mutation of NUDT5 releases the brake, boosting purine synthesis and giving cancer cells the fuel to resist treatment. Credit: Kilian Huber
<関連情報>
- https://www.ox.ac.uk/news/2025-11-07-discovery-reveals-handbrake-controls-cancer-drug-response
- https://www.science.org/doi/10.1126/science.adv4257
- https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c00072
プリンの新規合成を抑制するNudix加水分解酵素5の非酵素的役割 A non-enzymatic role of Nudix hydrolase 5 in repressing purine de novo synthesis
Tuan-Anh Nguyen, Jung-Ming G. Lin, Anne-Sophie M. C. Marques, Maximilian Fottner, […] , and Stefan Kubicek
Science Published:6 Nov 2025
DOI:https://doi.org/10.1126/science.adv4257
Abstract
Folate metabolism is intricately linked to purine de novo synthesis through the incorporation of folate-derived one-carbon units into the purine scaffold. By investigating chemical and genetic dependencies caused by mutations in methylenetetrahydrofolate dehydrogenase, cyclohydrolase and formyltetrahydrofolate synthetase 1 (MTHFD1), we discovered a key role for Nudix hydrolase 5 (NUDT5) in regulating purine de novo synthesis. Genetic depletion and selective chemical degradation showed that a scaffolding role, rather than NUDT5 enzymatic activity, was causing this phenotype. NUDT5 interacted with phosphoribosyl pyrophosphate amidotransferase (PPAT), the rate-limiting enzyme of purine de novo synthesis, to repress the pathway in response to increased purine abundance. Through this mechanism, loss of NUDT5 mediates resistance to purine analogs in cancer treatment and prevents adenosine toxicity in MTHFD1 deficiency.
臨床BTK阻害剤の予期せぬ非共有結合型オフターゲット活性がNUDT5/14二重拮抗薬の発見につながる Unexpected Noncovalent Off-Target Activity of Clinical BTK Inhibitors Leads to Discovery of a Dual NUDT5/14 Antagonist
Esra Balıkçı,Anne-Sophie M. C. Marques,Ludwig G. Bauer,Raina Seupel,James Bennett,Brigitt Raux,Karly Buchan,Klemensas Simelis,Usha Singh,Catherine Rogers,Jennifer Ward,Carol Cheng,Tamas Szommer,Kira Schützenhofer,Jonathan M. Elkins,David L. Sloman,Ivan Ahel,Oleg Fedorov,Paul E. Brennan,Kilian V. M. Huber,
Journal of Medicinal Chemistry Published: April 18, 2024
DOI:https://doi.org/10.1021/acs.jmedchem.4c00072
Abstract
Cofactor mimicry represents an attractive strategy for the development of enzyme inhibitors but can lead to off-target effects due to the evolutionary conservation of binding sites across the proteome. Here, we uncover the ADP-ribose (ADPr) hydrolase NUDT5 as an unexpected, noncovalent, off-target of clinical BTK inhibitors. Using a combination of biochemical, biophysical, and intact cell NanoBRET assays as well as X-ray crystallography, we confirm catalytic inhibition and cellular target engagement of NUDT5 and reveal an unusual binding mode that is independent of the reactive acrylamide warhead. Further investigation of the prototypical BTK inhibitor ibrutinib also revealed potent inhibition of the largely unstudied NUDIX hydrolase family member NUDT14. By exploring structure–activity relationships (SARs) around the core scaffold, we identify a potent, noncovalent, and cell-active dual NUDT5/14 inhibitor. Cocrystallization experiments yielded new insights into the NUDT14 hydrolase active site architecture and inhibitor binding, thus providing a basis for future chemical probe design.