2025-11-14 国立がん研究センター,慶應義塾大学,東北大学
<関連情報>
- https://www.ncc.go.jp/jp/information/pr_release/2025/1114/index.html
- https://www.ncc.go.jp/jp/information/pr_release/2025/1114/2025_1114.pdf
- https://www.nature.com/articles/s41467-025-64821-0
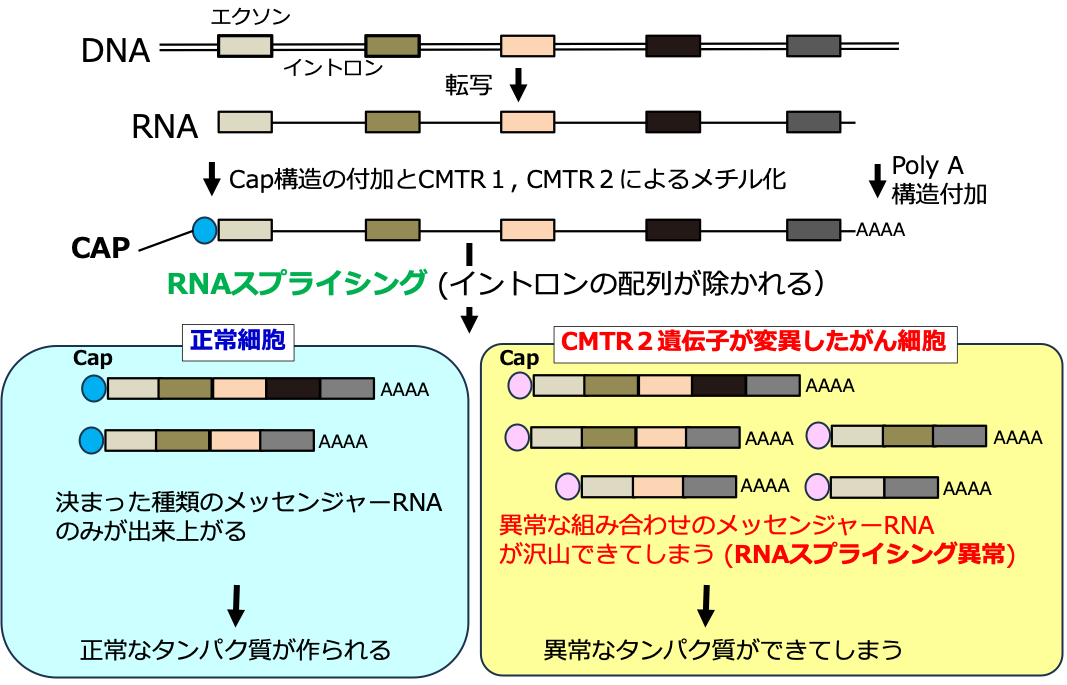
肺腺癌におけるCMTR2の変異はRNAの選択的スプライシングを変化させ、治療上の脆弱性を明らかにする Mutation of CMTR2 in Lung Adenocarcinoma Alters RNA Alternative Splicing and Reveals Therapeutic Vulnerabilities
Shigenari Nukaga,Kouya Shiraishi,Kenta Hamabe,Akifumi Mochizuki,Yu Hamaguchi,Emi Ogawa,Nguyen Thai Le,Yoko Shimada,Hanako Ono,Hitomi Nishinakamura,Yoshihisa Kobayashi,Junko Hamamoto,Ayako Ui,Mitsugu Araki,Yukari Sagae,Keiko Ohgino,Kai Sugihara,Satoshi Endo,Jun Miyakoshi,Yuichi Shiraishi,Hiroyuki Yasuda,Yasushi Okuno,Tatsuya Yoshida,Yasushi Goto,… Takashi Nakaoku
Nature Communications Published:06 November 2025
DOI:https://doi.org/10.1038/s41467-025-64821-0
Abstract
RNA splicing dysregulation has emerged as a hallmark of cancer and a promising therapeutic target; however, its full landscape in human solid cancer remains poorly characterized. To address this, we perform alternative splicing analyses using RNA-sequencing data from 751 lung adenocarcinoma samples from our cohort integrated with 519 samples from The Cancer Genome Atlas. Visualization of splicing patterns using t-distributed stochastic neighbor embedding reveals substantial inter-tumor heterogeneity driven by distinct molecular subtypes and histological differentiation. We identify a unique molecular subtype associated with inactivating mutations in CMTR2, which encodes Cap-specific mRNA (nucleoside-2’-O-)-methyltransferase 2. CMTR2 mutations are observed in 3.8% of cases and are predominantly truncating mutations, which form an isolated cluster within the splicing landscape. Intrinsic and CRISPR-Cas9-engineered CMTR2 mutations disrupt alternative splicing and sensitize cancer cells to sulfonamide-based RNA splicing modulators and immune checkpoint blockade therapy. Retrospective patient data confirm the increased sensitivity of CMTR2-deficient tumors to immune checkpoint blockade therapy. These findings uncover a previously unrecognized RNA splicing deficiency in human cancers and define a molecular subtype of lung adenocarcinoma driven by RNA splicing dysregulation, suggesting targets for therapeutic intervention in lung cancer.