2025-11-27 東京大学
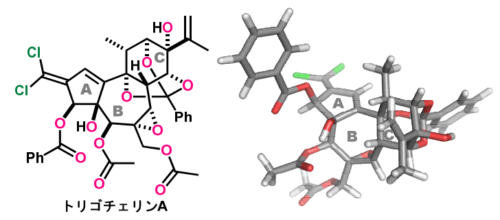
トリゴチェリン A の化学構造
<関連情報>
- https://www.u-tokyo.ac.jp/focus/ja/press/z0111_00094.html
- https://www.u-tokyo.ac.jp/content/400275360.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c17272
トリゴケリンAおよびCの全合成 Total Synthesis of Trigocherrins A and C
Kyohei Takaoka,Dan Matsubara,Manaka Matsumoto,Masanori Nagatomo,Koichi Hagiwara,and Masayuki Inoue
Journal of the American Chemical Society Published: November 25, 2025
DOI:https://doi.org/10.1021/jacs.5c17272
Abstract
Trigocherrins A (1) and C (2), isolated from an endangered tropical plant in New Caledonia, belong to a rare class of daphnane diterpenoid orthoesters with an unusual conjugated dichloroalkene moiety. Compound 1 displays antiviral activity against the chikungunya and dengue viruses. The fused 5/7/6-membered rings (ABC-rings) of 1 and 2 possess dichloromethylene, hydroxymethyl, and isopropenyl groups as the branched carbon chains, and epoxy, hydroxy, alkanoate, and C9,12,14-orthobenzoate groups as the oxygen functionalities. These exceedingly complex architectures with 11 contiguous stereocenters present multifaceted synthetic challenges. Here we report the first total synthesis of 1 and 2. Chemical assembly of 1 and 2 was achieved by designing new fragments and enabling a series of chemo- and stereoselective transformations. A fully substituted C-ring with the caged orthobenzoate was prepared from a starting d-ribose derivative and sequentially coupled with a four-carbon unit and an A-ring by radical and Stille reactions, respectively. An Ir(III)-catalyzed photoinduced decarboxylative radical reaction stereospecifically cyclized the seven-membered B-ring via the intermediacy of the bridgehead radical. The AB-rings were adjusted by constructing the dichloroalkene and installing the multiple oxygen functional groups through control of the intrinsic three-dimensional structures and reactivities of the intermediates. Ultimately, the total synthesis of 1 and 2 was achieved in 37 total steps. The new synthetic strategies and tactics described here provide valuable information for the syntheses of multicyclic natural products featuring diverse functional groups with varying reactivities.