2025-12-11 東京科学大学
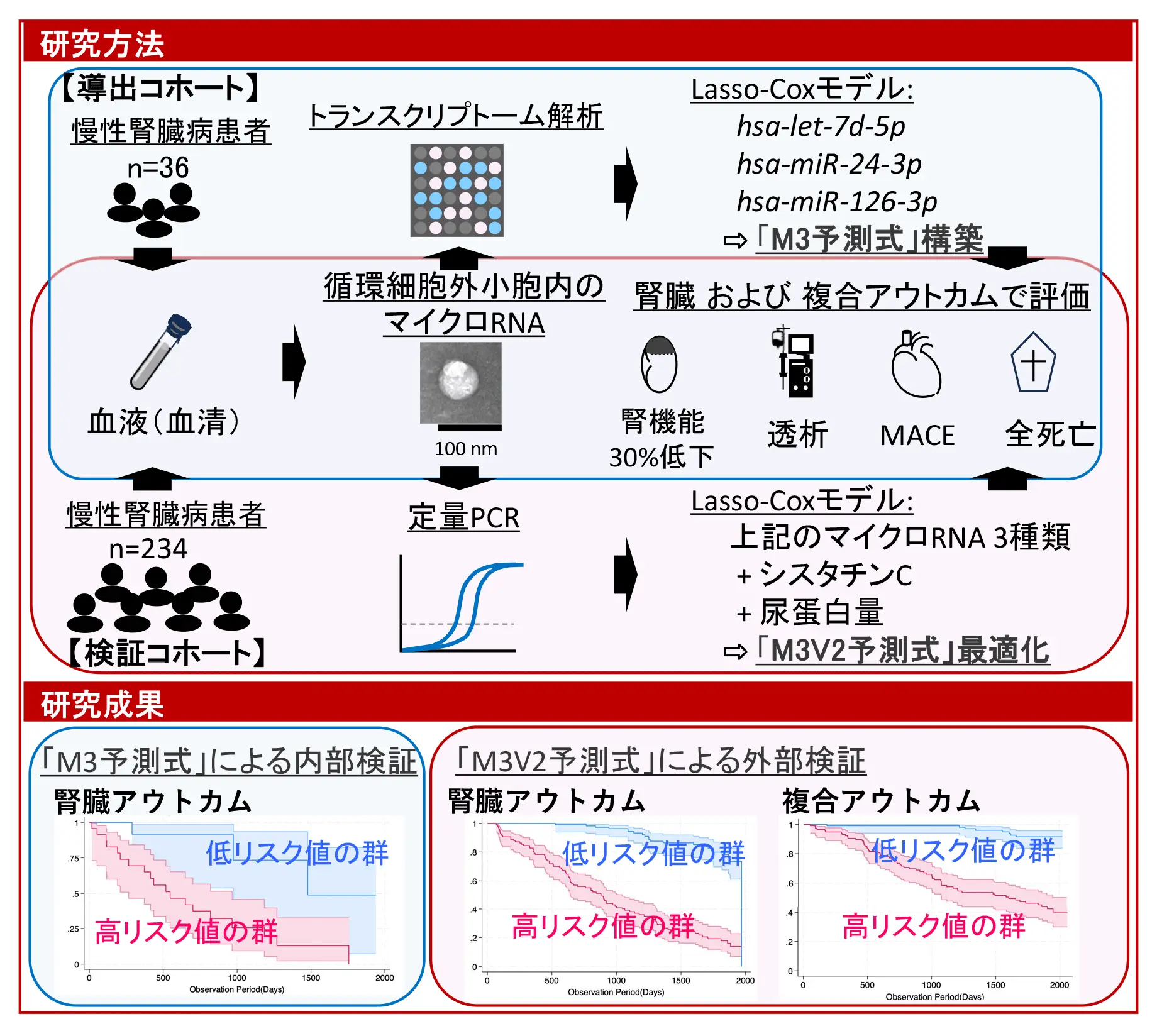
図1.本研究の構成と得られた成果の概要
<関連情報>
循環細胞外小胞マイクロRNAは腎臓および心血管イベントの予測バイオマーカーとして Circulating Extracellular Vesicle MicroRNAs as Predictive Biomarkers for Kidney and Cardiovascular Events
Shunsuke Inaba, MD, Takanori Hasegawa, PhD, Yuta Nakano, MD, PhD, Shotaro Naito, MD, PhD, Rena Suzukawa, Takaaki Koide, MD, PhD, Hisateru Sekiya, …, and Shintaro Mandai, MD, PhD
Journal of the American Heart Association Published: 10 December 2025
DOI:https://doi.org/10.1161/JAHA.125.045148
Abstract
Background
Chronic kidney disease (CKD) leads to premature mortality from cardiovascular events before kidney replacement therapy. Despite recognition of syndromes like cardiorenal anemia and cardiovascular‐kidney‐metabolic, predictive models for kidney and cardiovascular outcomes remain inadequate. This study aimed to develop a minimally invasive, risk model using circulating small extracellular vesicle‐derived miRNAs among patients with CKD.
Methods
A derivation cohort (n=36) underwent microarray‐based miRNA profiling, and a least absolute shrinkage and selection operator‐penalized Cox proportional hazards model was constructed. Validation was performed using TaqMan quantitative polymerase chain reaction in a cohort of 234 patients with CKD without kidney replacement therapy. The primary outcome was a ≥30% reduction in estimated glomerular filtration rate or progression to kidney replacement therapy. The secondary outcome included all‐cause mortality, kidney replacement therapy initiation, and major adverse cardiovascular events.
Results
In the derivation cohort, 36% of patients had hypertensive glomerulosclerosis as the underlying CKD cause, increasing to 48% in the validation cohort. Twenty‐three miRNAs were significantly downregulated in advanced CKD, associated with cellular senescence, FOXO (forkhead box, class O) signaling, and cell cycle pathways. From these, 3 miRNAs—hsa‐let‐7d‐5p, hsa‐miR‐24‐3p, and hsa‐miR‐126‐3p—were selected and integrated into the final risk score with cystatin C and urinary protein levels, following optimization in the validation cohort. Lower miRNA levels were linked to cardiovascular comorbidities and cardiorenal anemia syndrome. Over a median follow‐up of 39 and 59 months, 108 kidney events and 70 composite outcomes occurred. The model effectively predicted adverse outcomes across CKD causes, further stratifying risk within cardiovascular‐kidney‐metabolic stage classifications.
Conclusions
Circulating small extracellular vesicle‐derived miRNA profiles enable a noninvasive, longitudinally predictive model for adverse kidney and cardiovascular outcomes in CKD. This approach may improve early risk identification and clinical decision‐making.