2026-01-07 東京大学
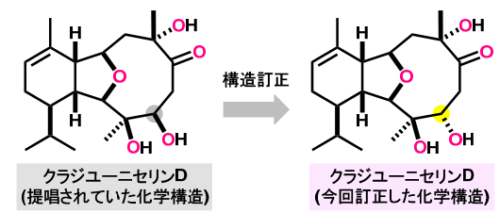
クラジユーニセリン D の化学構造と構造訂正
<関連情報>
- https://www.u-tokyo.ac.jp/focus/ja/press/z0111_00095.html
- https://www.u-tokyo.ac.jp/content/400277421.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c19112
12種のC4酸素化クラディエリンの一括全合成とクラディエユニセリンDおよびクラディエロイデスA/Cの構造解明 Collective Total Synthesis of 12 C4-Oxygenated Cladiellins and Structure Elucidation of Cladieunicellin D and Cladielloides A/C
Kyohei Oga,Yutaro Yamada,Masanori Nagatomo,Haruka Fujino,Masayuki Inoue
Journal of the American Chemical Society Published: December 30, 2025
DOI:https://doi.org/10.1021/jacs.5c19112
Abstract
Cladiellins are a group of marine natural products found in octocorals inhabiting Indo-Pacific reefs, and some members exhibit medically relevant biological activity. The 6/5/9-membered tricyclic ring skeleton of cladiellins comprises hydroisobenzofuran and oxonane moieties, and possesses three one-carbon branches and one three-carbon branch. Unsaturation and oxygenation at various positions endow structural diversity to this family; however, C4-oxygenated cladiellins are rare. Among these unusual structural features, the conformationally variable 9-membered ring presents an uncommon and formidable challenge for both NMR-based structure determination and chemical synthesis. Herein, we report the collective total synthesis of 12 structurally diverse C4-oxygenated cladiellins, 10 of which were chemically constructed for the first time. In addition, we revised the proposed structures of cladieunicellin D and cladielloide A, and established the previously unknown stereochemistry of cladielloides A/C. To accomplish this multifaceted endeavor, the project was divided into three parts. In the first part, we deduced the most likely stereoisomers of the three structurally ambiguous cladiellins from DP4+ probability analyses. In the second part, a common tricycle was assembled by a radical-polar crossover reaction of α-alkoxyacyl telluride with a 6-membered ring and an aldehyde, a reductive etherification reaction of a 5-membered ring, and a ring-closing metathesis reaction of a 9-membered ring. In the third part, the tricycle was systematically derivatized into 12 cladiellins by devising a series of selective transformations. The described results underscore the importance of designing an advanced common intermediate for collective total synthesis and combining density functional theory (DFT) calculations and syntheses for efficient structure elucidation.


