2026-01-08 国立がん研究センター,慶應義塾大学
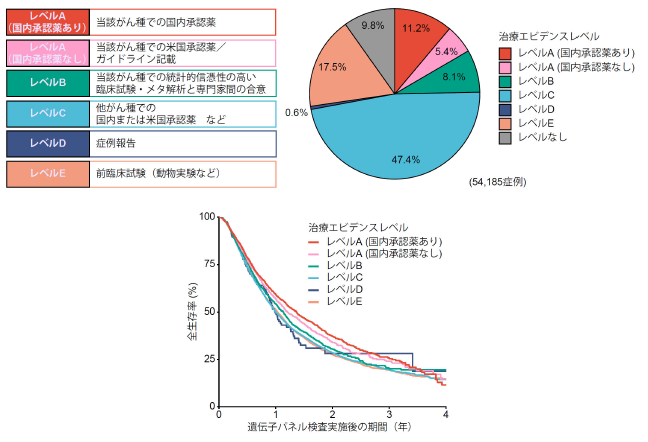
図1:(左上)治療エビデンスレベルの概略(詳細は注9参照)
(右上)全体における治療エビデンスレベルの割合
(下)治療エビデンスレベルごとの患者予後を示す生存曲線
<関連情報>
- https://www.ncc.go.jp/jp/information/pr_release/2026/0108/index.html
- https://www.ncc.go.jp/jp/information/pr_release/2026/0108/20260108.pdf
- https://www.nature.com/articles/s41591-025-04086-8
進行固形腫瘍における包括的ゲノムプロファイリングの実際の臨床的有用性 Real-world clinical utility of comprehensive genomic profiling in advanced solid tumors
Yuki Saito,Sara Horie,Yasunori Kogure,Kota Mizuno,Yuta Ito,Yosuke Mizukami,Haryoon Kim,Zen Tamura,Junji Koya,Takeru Funakoshi,Kenro Hirata & Keisuke Kataoka
Nature Medicine Published:06 January 2026
DOI:https://doi.org/10.1038/s41591-025-04086-8
Abstract
Comprehensive genomic profiling (CGP) is crucial in precision oncology, yet its real-world utility remains unclear. Here we analyzed data from the Japanese nationwide Center for Cancer Genomics and Advanced Therapeutics database, including clinical and genetic data from 54,185 patients with advanced solid tumors (consisting of 81 common and rare tumor types) who received CGP with a targeted sequencing panel covering 324 genes as part of their clinical care. We assessed the prognostic value of CGP-guided clinical evidence-level classification, showing that alterations predicting response to Pharmaceuticals and Medical Devices Agency-approved or Food and Drug Administration-approved therapies and to therapies supported by well-powered studies with expert consensus are detected in 16.6% and 8.1% of patients, respectively, and are associated with better prognosis than those with lower clinical evidence levels. Only 8% of patients receive CGP-guided approved–experimental genomic biomarker-linked therapies, although the proportion has improved over time. Substantial differences were observed across tumor types, with the proportions exceeding 20% in thyroid and lung cancers but remaining below 2% in pancreatic and liver cancers. Tumor-agnostic biomarker analyses reveal that tumor mutational burden (TMB) ≥20 mutations per megabase predicts better outcome across tumor types, regardless of microsatellite instability status, in TMB-high patients receiving pembrolizumab. Conversely, extramammary Pagetʼs disease is exceptionally resistant to pembrolizumab. The large-scale nationwide database allows evaluating inter-tumor type differences and investigating evidence-scarce situations, delineating where CGP offers greater benefit. These real-world findings complement those from clinical trials and prospective sequencing projects regarding CGP, providing valuable information for individualized treatment.