2026-02-02 ハーバード大学
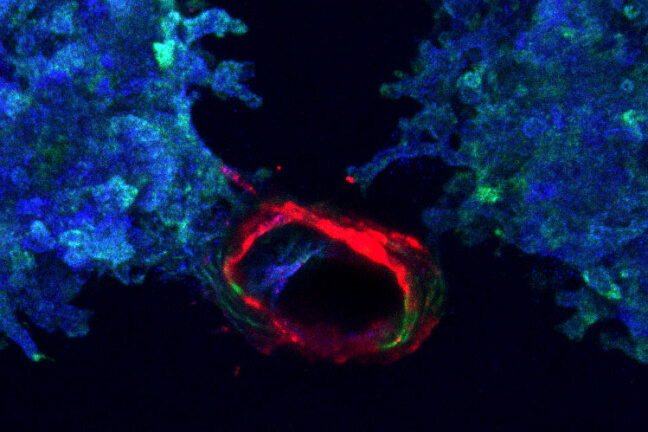
Printed ureteric tubule networks embedded in matrix, marked with green, connecting to a central, open ureteric bud-lined channel, marked with red.
<関連情報>
- https://seas.harvard.edu/news/toward-engineering-human-kidney-collecting-duct-system
- https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00288-0
尿管芽と集合管の灌流可能な3Dモデル Perfusable 3D models of ureteric bud and collecting duct tubules
Kayla J. Wolf ∙ Ronald C. van Gaal ∙ Sebastien G.M. Uzel ∙ … ∙ Paul Stankey ∙ Lisa M. Satlin ∙ Jennifer A. Lewis
Cell Biomaterials Published:December 18, 2025
DOI:https://doi.org/10.1016/j.celbio.2025.100297
The bigger picture
Recapitulating the complex architecture and function of human kidneys remains a major challenge in tissue engineering. Here, we leverage recent advances in generating ureteric bud (UB) and collecting duct (CD) organoids from human induced pluripotent stem cells (hiPSCs) to generate perfusable UB tubules that respond to luminal flow and exhibit budding reminiscent of early branching morphogenesis. We further extended this branching network by bioprinting UB cells in spatial arrangements that promote luminal fusion with the central perfusable channel, creating hierarchical tubular architectures. Finally, we transform these tubules into CD tubules under perfusive flow. Our work opens new avenues for drug testing, disease modeling, and creating bioengineered kidney tissues replete with CD networks for therapeutic use.
Highlights
- Perfusable human ureteric bud (UB) and collecting duct (CD) tubule models
- UB tubules that bud into a surrounding extracellular matrix
- Bioprinted UB networks that fuse with perfusable UB tubules
- Luminal flow applied during UB-to-CD differentiation enhances maturation
Summary
Recent protocols enable derivation of ureteric bud (UB) and collecting duct (CD) organoids from human induced pluripotent stem cells (hiPSCs), yet these organoids lack luminal flow and a drainage outlet. To address this, we created perfusable 3D models of UB and CD tubules. UB organoids were generated from hiPSCs, dissociated into single cells, and seeded onto a 3D perfusable channel embedded within extracellular matrix of fragmented basement membrane matrix and collagen I, where they self-assembled into a confluent monolayer. During perfusion, cells express UB markers over several weeks and undergo budding akin to early branching morphogenesis in developing kidneys. To promote network formation, UB cells were bioprinted adjacent to a perfusable UB tubule, forming interconnections through luminal fusion. Finally, perfused UB tubules were differentiated into CD tubules. Our platform facilitates understanding of human CD development while enabling future drug testing, disease modeling, and integration into therapeutic bioprinted kidney tissues.


