2026-02-06 清華大学
<関連情報>
- https://www.tsinghua.edu.cn/en/info/1245/14702.htm
- https://link.springer.com/article/10.1186/s13045-025-01773-4
細胞内空間アトラスは遠位胆管癌における神経周囲浸潤の微小環境リモデリングを明らかにする A subcellular spatial atlas illuminates the microenvironmental remodeling of perineural invasion in distal cholangiocarcinoma
Fansen Ji,Hao Chen,Huan Li,Jiawei Zhang,Sijia Li,Pengfei Wang,Hao Liu,Cui Ge,Bingjun Tang,Hongfang Yin,Xuedong Wang & Jiahong Dong
Journal of Hematology & Oncology Published:08 January 2026
DOI:https://doi.org/10.1186/s13045-025-01773-4
Abstract
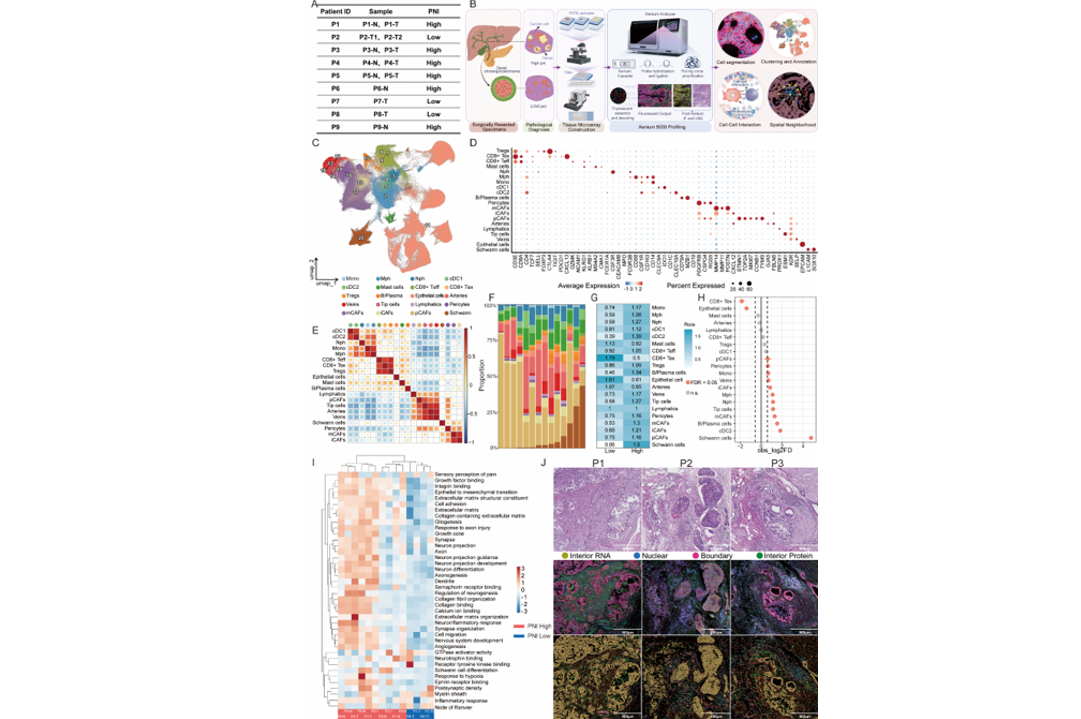
Distal cholangiocarcinoma (dCCA) arises from the distal bile duct and is anatomically embedded within the pancreatic head, adjacent to abundant autonomic nerve plexuses. This unique location renders dCCA particularly prone to perineural invasion (PNI), a pathological hallmark that contributes to its dismal prognosis. However, the spatial architecture and molecular drivers that orchestrate PNI remain poorly defined. Here, we applied Xenium subcellular resolution spatial transcriptomics platform to profile resected tumor tissues from dCCA patients stratified by PNI status pathologically. A spatially resolved atlas comprising a total of 20 cell types was generated, uncovering enrichment of Schwann cells, type 2 conventional dendritic cells (cDC2), M2-like macrophages, cancer associated fibroblasts (CAFs) and B/plasma cells in PNI-high tumors, along with depletion of exhausted CD8+ T cells. Heterogeneous malignant cells in PNI-high tumors demonstrated activation of extracellular matrix remodeling and axonogenesis pathways, in line with the initial pathological classification. Spatial mapping further revealed distinct PNI-associated niches, notably matrix-producing CAFs (mCAFs)-macrophage clusters exhibiting coordinated enrichment of inflammatory and fibrotic programs. We further identified the LAMB3-DAG1 axis as a potential mediator of dCCA cells-Schwann cell interaction, while the preferential proximity of arteries to Schwann cells suggested additional microenvironmental support for nerve invasion. Collectively, our study provides a comprehensive subcellular atlas of PNI in dCCA, uncovering coordinated epithelial, stromal, and immune remodeling that drives perineural invasion. The identified biomarkers not only hold promise for patient stratification but may also guide intraoperative navigation and surgical margin determination, offering new avenues for precision therapy.