2026-02-09 東京科学大学
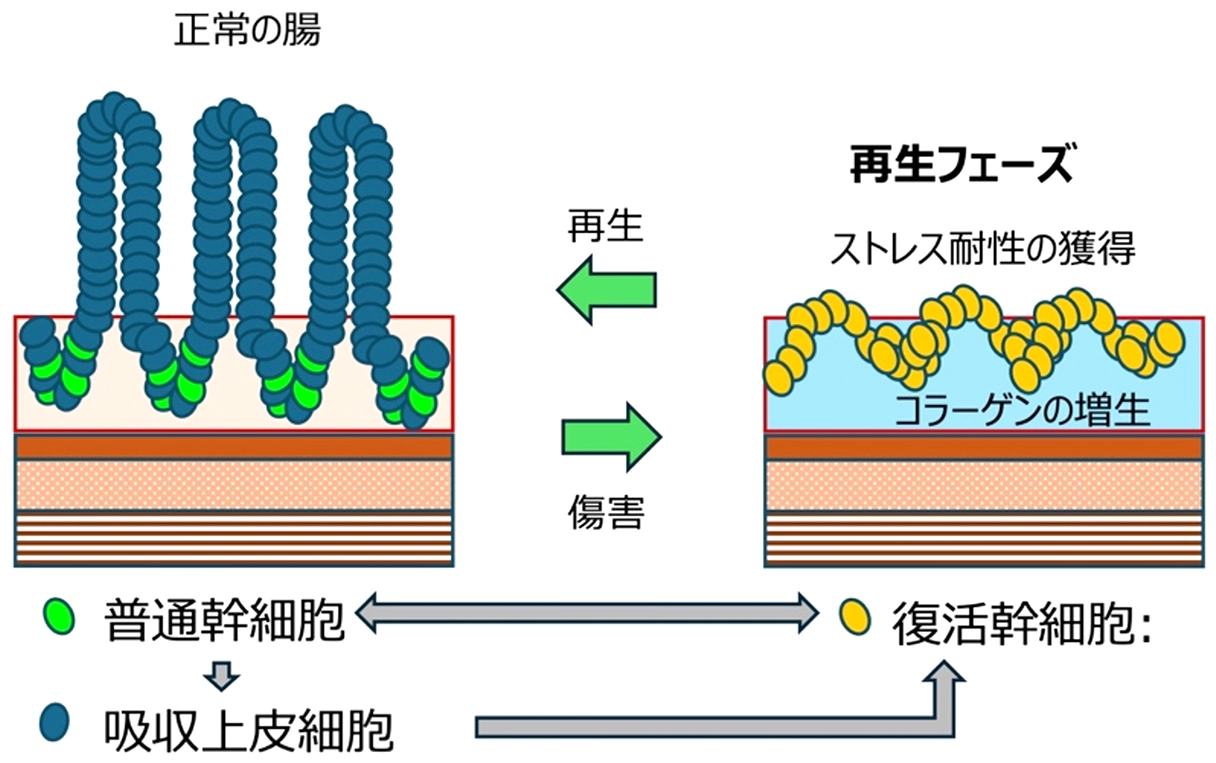
図1. 腸の普通幹細胞と復活幹細胞の可逆的な関係。
普通幹細胞から分化した吸収上皮細胞は、傷害時に復活幹細胞へと状態転換し、最終的に普通幹細胞へと戻る。復活幹細胞は高いストレス耐性を有する。
<関連情報>
- https://www.isct.ac.jp/ja/news/aminlemao62n
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=3190&prevId=&key=52dcfdad8cbb683d52a89e7ecb3fd0e8.pdf
- https://www.nature.com/articles/s42003-026-09533-x
多様な系統からの胎児復帰は腸管幹細胞プールを維持し、ストレス耐性を付与する Fetal reversion from diverse lineages sustains the intestinal stem cell pool and confers stress resilience
Sakura Kirino,Fumiya Uefune,Kensuke Miyake,Nobuhiko Ogasawara,Sakurako Kobayashi,Satoshi Watanabe,Yui Hiraguri,Go Ito,Keiichi Akahoshi,Daisuke Ban,Johan H. van Es,Hans Clevers,Mamoru Watanabe,Ryuichi Okamoto & Shiro Yui
Communications Biology Published:13 January 2026
DOI:https://doi.org/10.1038/s42003-026-09533-x
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
Abstract
Plasticity is a central mechanism underlying the robust regenerative capacity of the intestinal epithelium. Two major forms of plasticity have been described: spatial plasticity, in which differentiated cells revert to crypt base columnar cells (CBCs), and fetal reversion into revival stem cells (revSCs). However, the relationship among these two stem cell populations and differentiated cells remains to be clarified. Here, we demonstrated the bidirectional interconversion between CBCs and revSCs. Using lineage tracing, injury models and villus culture, we show that absorptive enterocytes can reprogram into revSCs and regenerate CBCs. These findings position fetal reversion as an entry point to spatial plasticity, establishing a regenerative hierarchy where CBCs, revSCs, and enterocytes collectively orchestrate intestinal repair. Furthermore, we identified revSCs as a highly stress-tolerant stem cell population, whose emergence would preserve the stem cell pool. Our results establish fetal reversion as a cellular escape mechanism safeguarding epithelial regeneration under inflammatory conditions.