非侵襲的で痛みのない治療法は、化学療法を補完し、副作用を軽減する可能性があります。 Non-invasive and painless treatment complements chemotherapy and potentially lowers adverse side effects
2022-05-03 シンガポール国立大学(NUS)
シンガポール国立大学(NUS)の研究チームは、乳がんの治療効果を高めるために化学療法の効果的な併用療法として、OncoFTXシステムによる新しい磁気療法を開拓しています。
磁気治療では、1セッションあたり3ミリテスラの強さのパルス磁場を1時間、乳房に当てます。この磁場の大きさは、地球磁場の約50倍であり、従来の磁気共鳴イメージングと比べると1000分の1です。差し迫った安全性と有効性の試験により、乳がん患者にとって最適な治療頻度が決定されます。
<関連情報>
- https://news.nus.edu.sg/magnetic-therapy-enhances-chemotherapy-treatment-of-breast-cancer/
- https://www.frontiersin.org/articles/10.3389/fonc.2021.783803/full
TRPC1の発現量の変化により、乳がんのドキソルビシンおよび磁場治療に対する感受性が予測される。プレシジョン・メディシン・アプローチの実現へ向けて Modulated TRPC1 Expression Predicts Sensitivity of Breast Cancer to Doxorubicin and Magnetic Field Therapy: Segue Towards a Precision Medicine Approach
Yee Kit Tai, Karen Ka Wing Chan, Charlene Hui Hua Fong, Sharanya Ramanan, Jasmine Lye Yee Yap, Jocelyn Naixin Yin, Yun Sheng Yip, Wei Ren Tan, Angele Pei Fern Koh, Nguan Soon Tan, Ching Wan Chan, Ruby Yun Ju Huang, Jing Ze Li, Jürg Fröhlich and Alfredo Franco-Obregón
Frontiersin Oncology Pblished:24 January 2022
DOI:https://doi.org/10.3389/fonc.2021.783803
Abstract
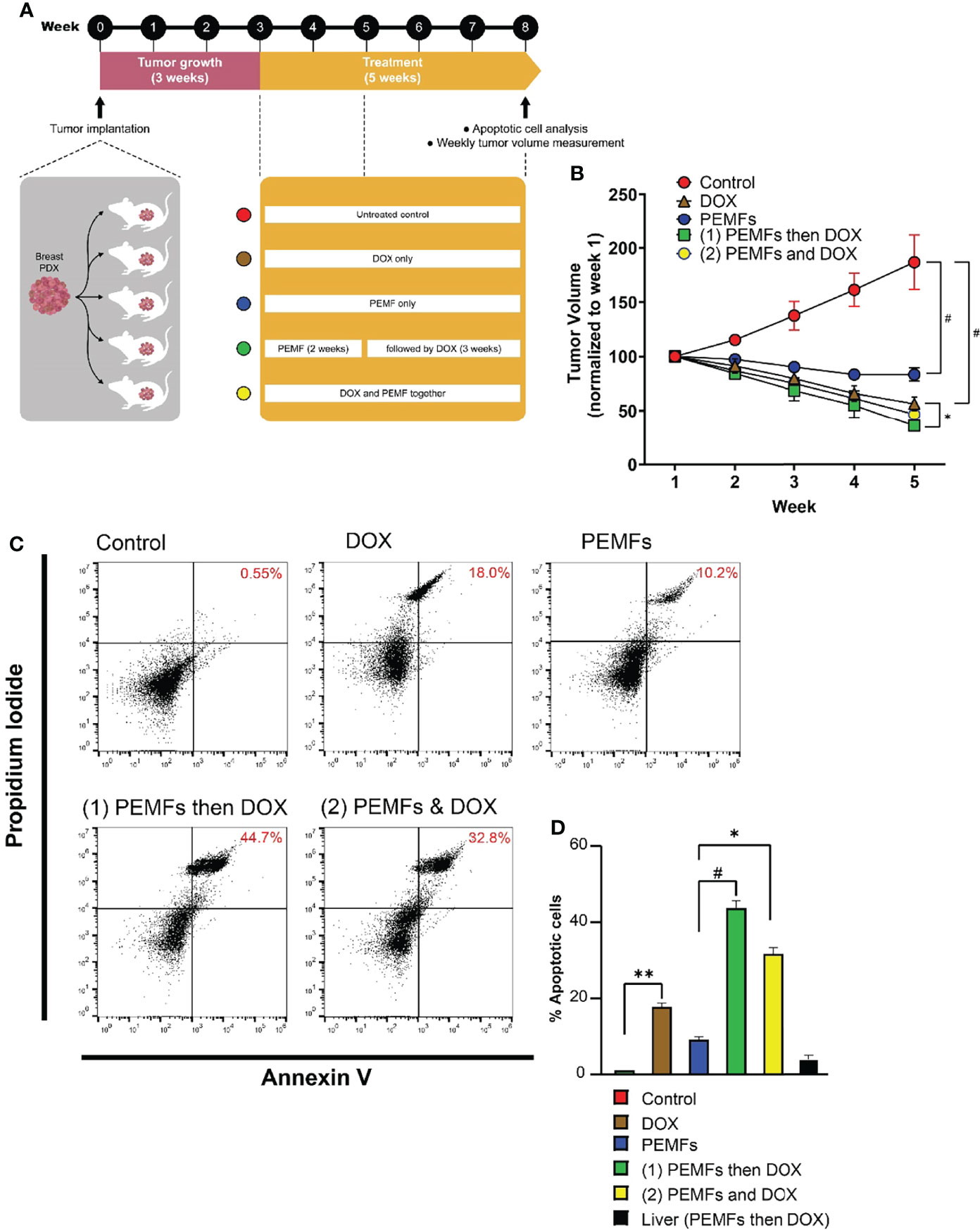
Chemotherapy is the mainstream treatment modality for invasive breast cancer. Unfortunately, chemotherapy-associated adverse events can result in early termination of treatment. Paradoxical effects of chemotherapy are also sometimes observed, whereby prolonged exposure to high doses of chemotherapeutic agents results in malignant states resistant to chemotherapy. In this study, potential synergism between doxorubicin (DOX) and pulsed electromagnetic field (PEMF) therapy was investigated in: 1) MCF-7 and MDA-MB-231 cells in vitro; 2) MCF-7 tumors implanted onto a chicken chorioallantoic membrane (CAM) and; 3) human patient-derived and MCF-7 and MDA-MB-231 breast cancer xenografts implanted into NOD-SCID gamma (NSG) mice. In vivo, synergism was observed in patient-derived and breast cancer cell line xenograft mouse models, wherein PEMF exposure and DOX administration individually reduced tumor size and increased apoptosis and could be augmented by combined treatments. In the CAM xenograft model, DOX and PEMF exposure also synergistically reduced tumor size as well as reduced Transient Receptor Potential Canonical 1 (TRPC1) channel expression. In vitro, PEMF exposure alone impaired the survival of MCF-7 and MDA-MB-231 cells, but not that of non-malignant MCF10A breast cells; the selective vulnerability of breast cancer cells to PEMF exposure was corroborated in human tumor biopsy samples. Stable overexpression of TRPC1 enhanced the vulnerability of MCF-7 cells to both DOX and PEMF exposure and promoted proliferation, whereas TRPC1 genetic silencing reduced sensitivity to both DOX and PEMF treatments and mitigated proliferation. Chronic exposure to DOX depressed TRPC1 expression, proliferation, and responses to both PEMF exposure and DOX in a manner that was reversible upon removal of DOX. TRPC1 channel overexpression and silencing positively correlated with markers of epithelial-mesenchymal transition (EMT), including SLUG, SNAIL, VIMENTIN, and E-CADHERIN, indicating increased and decreased EMT, respectively. Finally, PEMF exposure was shown to attenuate the invasiveness of MCF-7 cells in correlation with TRPC1 expression. We thus demonstrate that the expression levels of TRPC1 consistently predicted breast cancer sensitivity to DOX and PEMF interventions and positively correlated to EMT status, providing an initial rationale for the use of PEMF-based therapies as an adjuvant to DOX chemotherapy for the treatment of breast cancers characterized by elevated TRPC1 expression levels.