2022-05-19 ピッツバーグ大学
<関連情報>
- https://news.engineering.pitt.edu/pitt-physician-scientist-pushes-for-prognostic-test-to-end-silent-epidemic/
- https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2792378
敗血症に伴う急性腎不全の病期分類におけるバイオマーカーの有用性 Utility of Biomarkers for Sepsis-Associated Acute Kidney Injury Staging
Luca Molinari, Gaspar Del Rio-Pertuz, Ali Smith, Douglas P. Landsittel, Kai Singbartl, Paul M. Palevsky, Lakhmir S. Chawla, MD9; David T. Huang, MD4,10; Donald M. Yealy, MD10; Derek C. Angus, MD4; John A. Kellum, for the ProCESS and ProGReSS-AKI Investigators
JAMA Network Open Published:May 18, 2022
DOI:10.1001/jamanetworkopen.2022.12709
Abstract
Importance The 23rd Acute Disease Quality Initiative (ADQI-23) consensus conference proposed a framework to integrate biomarkers into the staging of acute kidney injury (AKI). It is unknown whether tissue inhibitor of metalloproteinases 2 (TIMP-2) and insulinlike growth factor binding protein 7 (IGFBP7) could be used for staging.
Objective To test whether higher levels of urinary [TIMP-2] × [IGFBP7] are associated with lower survival among patients with the same functional stage of AKI.
Design, Setting, and Participants This cohort study was performed using data from the Protocolized Care for Early Septic Shock (ProCESS) trial, which enrolled critically ill patients with septic shock who presented at academic and community emergency departments and intensive care units in the US from March 2008 to May 2013. Patients with end-stage kidney disease, a reference serum creatinine level of 4 mg/dL or greater (to convert to μmol/L, multiply by 76.25), or missing data on serum creatinine levels or urinary levels of [TIMP-2] × [IGFBP7] were excluded. Data were analyzed from October 2020 to October 2021.
Exposures The presence of AKI, assessed using Kidney Disease: Improving Global Outcomes criteria within 24 hours after enrollment and the highest AKI stage as well as urinary [TIMP-2] × [IGFBP7] level at 6 hours after enrollment. A previously reported high-specificity cutoff level for [TIMP-2] × [IGFBP7] of 2.0 (ng/mL)2/1000 was used to categorize patients (including those without functional criteria of AKI) according to the new staging system proposed by the ADQI-23 as biomarker negative (urinary [TIMP-2] × [IGFBP7] level ≤2.0 [ng/mL]2/1000) or biomarker positive ([TIMP-2] × [IGFBP7] >2.0 [ng/mL]2/1000).
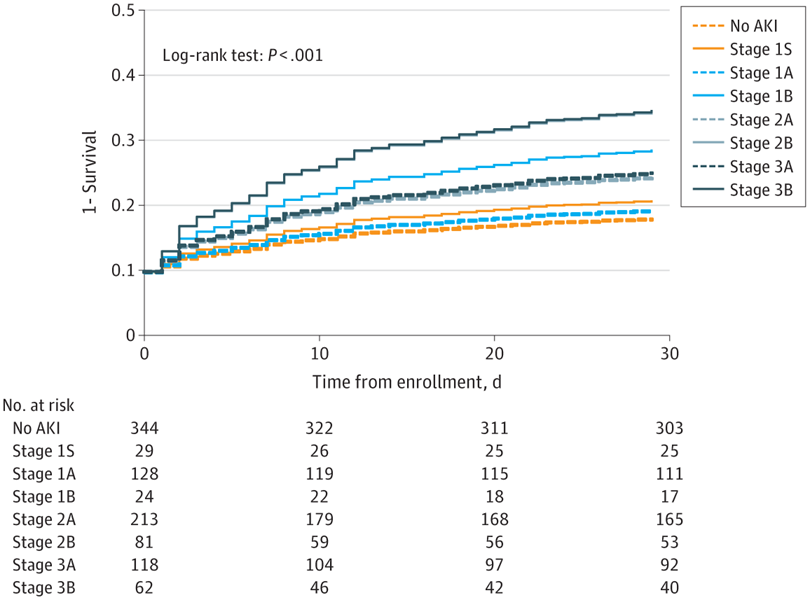
Main Outcomes and Measures Survival (assessed using Kaplan-Meier plots and the log-rank test) and mortality (assessed using relative risk [RR] 30 days after enrollment).
Results The analysis included 999 patients with a median age of 61 years (IQR, 50-73 years); 554 (55.5%) were male. Biomarker-positive patients had lower survival and higher mortality at 30 days in the groups with AKI stage 1 (RR, 2.20; 95% CI, 1.02-4.72), stage 2 (RR, 1.53; 95% CI, 1.04-2.27), and stage 3 (RR, 1.61; 95% CI, 1.00-2.60). The associations were specific to patients with AKI. No difference in 30-day survival was found between biomarker-positive and biomarker-negative patients in the absence of functional criteria for AKI (RR, 1.16; 95% CI, 0.45-3.01).
Conclusions and Relevance The findings suggest that assessment of the cell-cycle arrest biomarkers TIMP-2 and IGFBP7 may augment AKI staging for patients with functional criteria for AKI.