変異によりスパイクタンパク質がより硬くなり、ウイルスのフィットネスを向上させる可能性がある。 Mutations made spike protein more rigid, potentially improving virus’ fitness
2023-03-31 ペンシルベニア州立大学(PennState)
また、Omicron変異株のスパイクタンパク質の中心部は、可能な限り硬くなったため、より新しいワクチンは元の変異株を対象にしたものよりも長期間効果的である可能性がある。
<関連情報>
- https://www.psu.edu/news/research/story/tighter-core-stabilizes-sars-cov-2-spike-protein-new-emergent-variants/
- https://elifesciences.org/articles/82584
出現した SARS-CoV-2 変異体におけるスパイク構造ダイナミクスの変化のタイムラインは、変化した NTD ダイナミクスによる三量体ストークの漸進的な安定化を明らかにする Timeline of changes in spike conformational dynamics in emergent SARS-CoV-2 variants reveal progressive stabilization of trimer stalk with altered NTD dynamics
Sean M Braet,Theresa SC Buckley,Varun Venkatakrishnan,Kim-Marie A Dam,Pamela J Bjorkman,Ganesh S Anand
eLife Published:Mar 17, 2023
DOI:https://doi.org/10.7554/eLife.82584
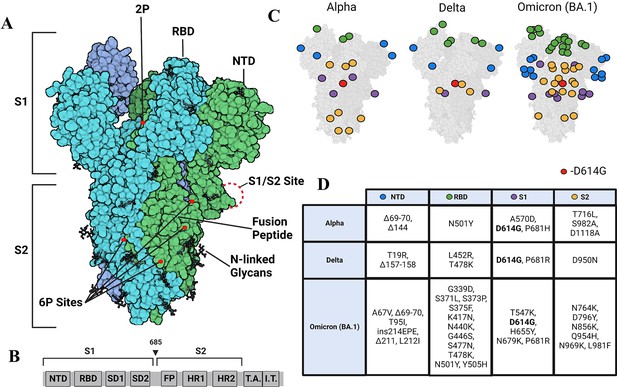
Abstract
SARS-CoV-2 emergent variants are characterized by increased viral fitness and each shows multiple mutations predominantly localized to the spike (S) protein. Here, amide hydrogen/deuterium exchange mass spectrometry has been applied to track changes in S dynamics from multiple SARS-CoV-2 variants. Our results highlight large differences across variants at two loci with impacts on S dynamics and stability. A significant enhancement in stabilization first occurred with the emergence of D614G S followed by smaller, progressive stabilization in subsequent variants. Stabilization preceded altered dynamics in the N-terminal domain, wherein Omicron BA.1 S showed the largest magnitude increases relative to other preceding variants. Changes in stabilization and dynamics resulting from S mutations detail the evolutionary trajectory of S in emerging variants. These carry major implications for SARS-CoV-2 viral fitness and offer new insights into variant-specific therapeutic development.