2023-05-03 カリフォルニア大学バークレー校(UCB)
◆深い睡眠は、βアミロイドタンパク質の蓄積を早める不良な睡眠とは異なり、認知機能の維持に役立つとされる。研究により、既にアルツハイマー病の高い病理量を持つ人々が十分な量の深い睡眠を取ることで、記憶低下から保護されることが明らかになった。
◆これは、認知予備力に関する研究に大きな前進をもたらす可能性がある。そして、年齢を重ねるベビーブーマー世代にとっては、この研究がアルツハイマー病との戦いに役立つことが期待される。
<関連情報>
- https://news.berkeley.edu/2023/05/03/deep-sleep-may-mitigate-alzheimers-memory-loss-berkeley-research-shows/
- https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-023-02811-z
アルツハイマー病の病態に直面した際の新規保護的認知予備因子としてのNREM睡眠 NREM sleep as a novel protective cognitive reserve factor in the face of Alzheimer’s disease pathology
Zsófia Zavecz,Vyoma D. Shah,Olivia G. Murillo,Raphael Vallat,Bryce A. Mander,Joseph R. Winer,William J. Jagust & Matthew P. Walker
BMC Medicine Published:03 May 2023
DOI:https://doi.org/10.1186/s12916-023-02811-z
Abstract
Background
Alzheimer’s disease (AD) pathology impairs cognitive function. Yet some individuals with high amounts of AD pathology suffer marked memory impairment, while others with the same degree of pathology burden show little impairment. Why is this? One proposed explanation is cognitive reserve i.e., factors that confer resilience against, or compensation for the effects of AD pathology. Deep NREM slow wave sleep (SWS) is recognized to enhance functions of learning and memory in healthy older adults. However, that the quality of NREM SWS (NREM slow wave activity, SWA) represents a novel cognitive reserve factor in older adults with AD pathology, thereby providing compensation against memory dysfunction otherwise caused by high AD pathology burden, remains unknown.
Methods
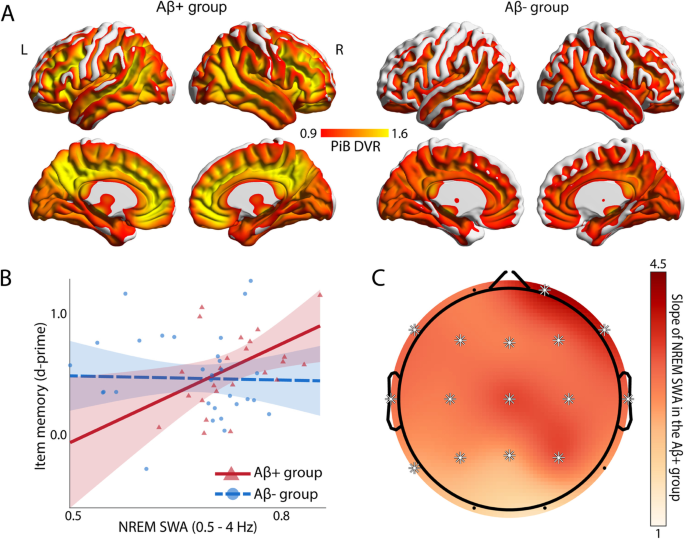
Here, we tested this hypothesis in cognitively normal older adults (N = 62) by combining 11C-PiB (Pittsburgh compound B) positron emission tomography (PET) scanning for the quantification of β-amyloid (Aβ) with sleep electroencephalography (EEG) recordings to quantify NREM SWA and a hippocampal-dependent face-name learning task.
Results
We demonstrated that NREM SWA significantly moderates the effect of Aβ status on memory function. Specifically, NREM SWA selectively supported superior memory function in individuals suffering high Aβ burden, i.e., those most in need of cognitive reserve (B = 2.694, p = 0.019). In contrast, those without significant Aβ pathological burden, and thus without the same need for cognitive reserve, did not similarly benefit from the presence of NREM SWA (B = -0.115, p = 0.876). This interaction between NREM SWA and Aβ status predicting memory function was significant after correcting for age, sex, Body Mass Index, gray matter atrophy, and previously identified cognitive reserve factors, such as education and physical activity (p = 0.042).
Conclusions
These findings indicate that NREM SWA is a novel cognitive reserve factor providing resilience against the memory impairment otherwise caused by high AD pathology burden. Furthermore, this cognitive reserve function of NREM SWA remained significant when accounting both for covariates, and factors previously linked to resilience, suggesting that sleep might be an independent cognitive reserve resource. Beyond such mechanistic insights are potential therapeutic implications. Unlike many other cognitive reserve factors (e.g., years of education, prior job complexity), sleep is a modifiable factor. As such, it represents an intervention possibility that may aid the preservation of cognitive function in the face of AD pathology, both present moment and longitudinally.


