2024-04-10 マックス・プランク研究所
<関連情報>
- https://www.mpg.de/21811459/0410-terr-discovery-of-the-first-fractal-molecule-in-nature-153410-x
- https://www.nature.com/articles/s41586-024-07287-2
代謝酵素の進化におけるフラクタル幾何学の出現 Emergence of fractal geometries in the evolution of a metabolic enzyme
Franziska L. Sendker,Yat Kei Lo,Thomas Heimerl,Stefan Bohn,Louise J. Persson,Christopher-Nils Mais,Wiktoria Sadowska,Nicole Paczia,Eva Nußbaum,María del Carmen Sánchez Olmos,Karl Forchhammer,Daniel Schindler,Tobias J. Erb,Justin L. P. Benesch,Erik G. Marklund,Gert Bange,Jan M. Schuller & Georg K. A. Hochberg
Nature Published:10 April 2024
DOI:https://doi.org/10.1038/s41586-024-07287-2
Abstract
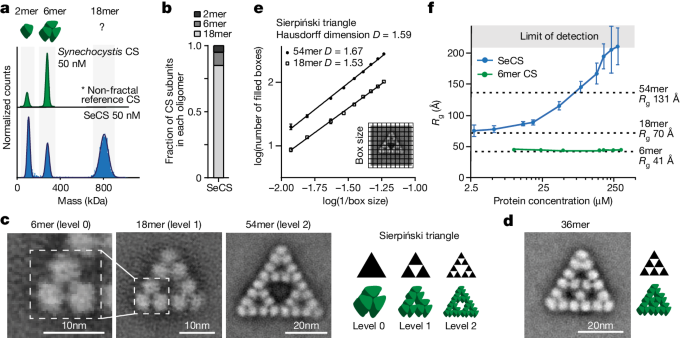
Fractals are patterns that are self-similar across multiple length-scales1. Macroscopic fractals are common in nature2,3,4; however, so far, molecular assembly into fractals is restricted to synthetic systems5,6,7,8,9,10,11,12. Here we report the discovery of a natural protein, citrate synthase from the cyanobacterium Synechococcus elongatus, which self-assembles into Sierpiński triangles. Using cryo-electron microscopy, we reveal how the fractal assembles from a hexameric building block. Although different stimuli modulate the formation of fractal complexes and these complexes can regulate the enzymatic activity of citrate synthase in vitro, the fractal may not serve a physiological function in vivo. We use ancestral sequence reconstruction to retrace how the citrate synthase fractal evolved from non-fractal precursors, and the results suggest it may have emerged as a harmless evolutionary accident. Our findings expand the space of possible protein complexes and demonstrate that intricate and regulatable assemblies can evolve in a single substitution.