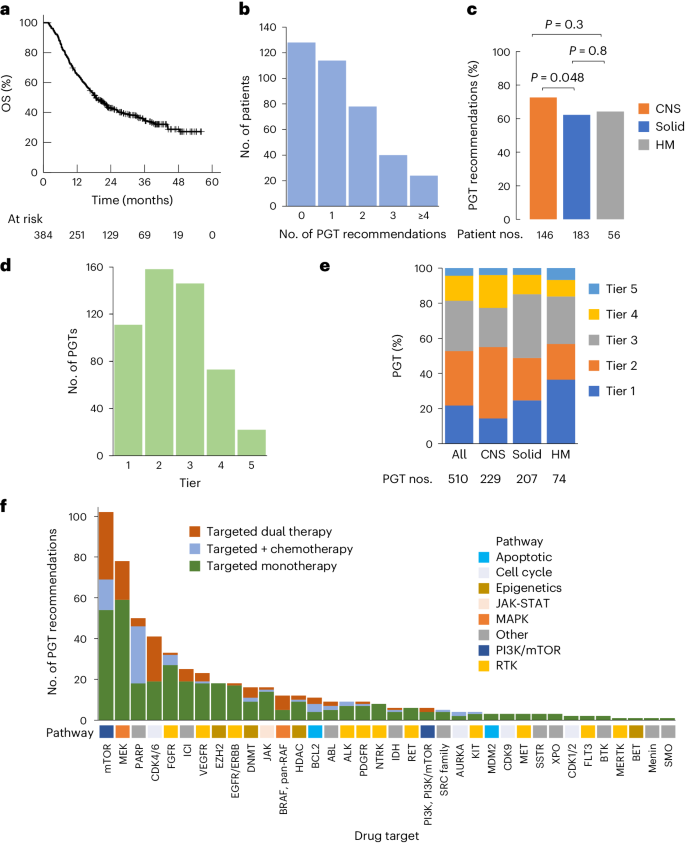
2024-05-24 ニューサウスウェールズ大学(UNSW)
 A weekly dose of semaglutide reduced the risk of major kidney events by 24 per cent, a clinical trial has found.Photo: Adobe/Fernanda Carrasco Maldonado
A weekly dose of semaglutide reduced the risk of major kidney events by 24 per cent, a clinical trial has found.Photo: Adobe/Fernanda Carrasco Maldonado
<関連情報>
- https://www.unsw.edu.au/newsroom/news/2024/05/Diabetes-medication-semaglutide-found-to-reduce-risk-of-kidney-disease-and-death
- https://www.nejm.org/doi/full/10.1056/NEJMoa2403347
2型糖尿病患者の慢性腎臓病に対するセマグルチドの効果 Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes
Vlado Perkovic, M.B., B.S., Ph.D., Katherine R. Tuttle, M.D. , Peter Rossing, M.D., D.M.Sc., Kenneth W. Mahaffey, M.D., Johannes F.E. Mann, M.D., George Bakris, M.D., Florian M.M. Baeres, M.D., Thomas Idorn, M.D., Ph.D., Heidrun Bosch-Traberg, M.D., Nanna Leonora Lausvig, M.Sc., and Richard Pratley, M.D., for the FLOW Trial Committees and Investigators
The New England Journal of Medicine Published May 24, 2024
DOI: 10.1056/NEJMoa2403347
Abstract
BACKGROUND
Patients with type 2 diabetes and chronic kidney disease are at high risk for kidney failure, cardiovascular events, and death. Whether treatment with semaglutide would mitigate these risks is unknown.
METHODS
We randomly assigned patients with type 2 diabetes and chronic kidney disease (defined by an estimated glomerular filtration rate [eGFR] of 50 to 75 ml per minute per 1.73 m2 of body-surface area and a urinary albumin-to-creatinine ratio [with albumin measured in milligrams and creatinine measured in grams] of >300 and <5000 or an eGFR of 25 to <50 ml per minute per 1.73 m2 and a urinary albumin-to-creatinine ratio of >100 and <5000) to receive subcutaneous semaglutide at a dose of 1.0 mg weekly or placebo. The primary outcome was major kidney disease events, a composite of the onset of kidney failure (dialysis, transplantation, or an eGFR of <15 ml per minute per 1.73 m2), at least a 50% reduction in the eGFR from baseline, or death from kidney-related or cardiovascular causes. Prespecified confirmatory secondary outcomes were tested hierarchically.
RESULTS
Among the 3533 participants who underwent randomization (1767 in the semaglutide group and 1766 in the placebo group), median follow-up was 3.4 years, after early trial cessation was recommended at a prespecified interim analysis. The risk of a primary-outcome event was 24% lower in the semaglutide group than in the placebo group (331 vs. 410 first events; hazard ratio, 0.76; 95% confidence interval [CI], 0.66 to 0.88; P=0.0003). Results were similar for a composite of the kidney-specific components of the primary outcome (hazard ratio, 0.79; 95% CI, 0.66 to 0.94) and for death from cardiovascular causes (hazard ratio, 0.71; 95% CI, 0.56 to 0.89). The results for all confirmatory secondary outcomes favored semaglutide: the mean annual eGFR slope was less steep (indicating a slower decrease) by 1.16 ml per minute per 1.73 m2 in the semaglutide group (P<0.001), the risk of major cardiovascular events 18% lower (hazard ratio, 0.82; 95% CI, 0.68 to 0.98; P=0.029), and the risk of death from any cause 20% lower (hazard ratio, 0.80; 95% CI, 0.67 to 0.95, P=0.01). Serious adverse events were reported in a lower percentage of participants in the semaglutide group than in the placebo group (49.6% vs. 53.8%).
CONCLUSIONS
Semaglutide reduced the risk of clinically important kidney outcomes and death from cardiovascular causes in patients with type 2 diabetes and chronic kidney disease. (Funded by Novo Nordisk; FLOW ClinicalTrials.gov number, NCT03819153.)