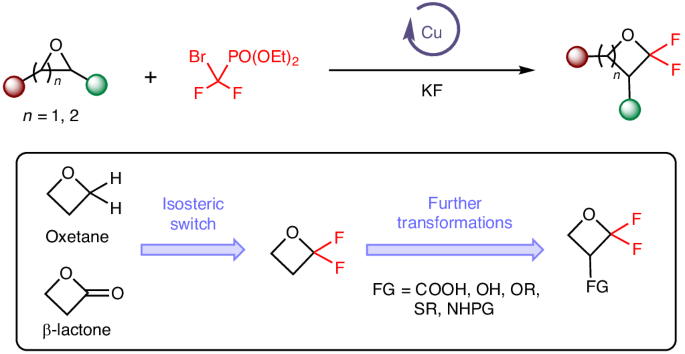
2025-02-21 ゲーテ大学
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/loss-of-the-y-chromosome-discovered-as-a-new-risk-factor-for-heart-disease/
- https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaf035/8009275
Y染色体のモザイク欠損と冠動脈造影後の死亡率 Mosaic loss of Y chromosome and mortality after coronary angiography
Michael Weyrich, Stephen Zewinger, Tamim Sarakpi, Tina Rasper, Marcus E Kleber, Sebastian Cremer, Lukas Zanders, Fenja Fleck, Agneta Siegbahn, Lars Wallentin, …
European Heart Journal Published:12 February 2025
DOI:https://doi.org/10.1093/eurheartj/ehaf035
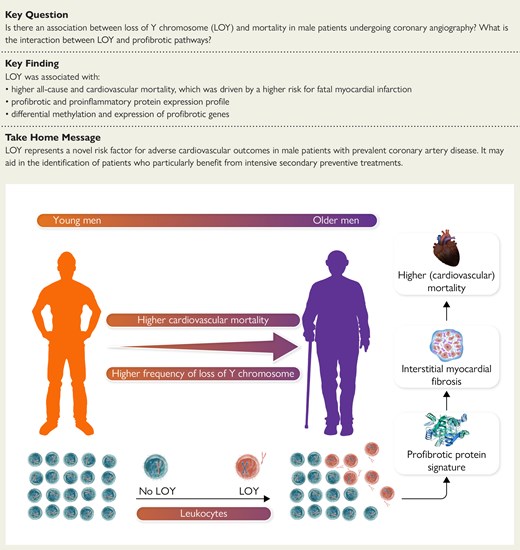
structured graphical abstract
Frequency of loss of Y chromosome (LOY) in leukocytes increases with age, associates with a profibrotic protein signature, interstitial myocardial fibrosis, and leads to higher cardiovascular mortality.
Abstract
Background and Aims
Acquired somatic mutations emerged as important drivers of adverse cardiovascular disease outcomes. Recently, mosaic loss of Y chromosome (LOY) in haematopoietic cells was identified to induce diffuse cardiac fibrosis in male mice. The aim of the present study was to determine the association between LOY and cardiovascular mortality in patients undergoing coronary angiography.
Methods
LOY was quantified in 1698 male participants of the LURIC study, who underwent coronary angiography, and its association with all-cause and cardiovascular mortality was determined. Furthermore, the interaction between LOY and inherited genetic susceptibility for cardiac fibrosis was assessed.
Results
The frequency of LOY steeply increased in male participants of LURIC at the age of 60 years. Loss of Y chromosome > 17% was associated with significantly higher all-cause [hazard ratio (HR) 1.41, 95% confidence interval (CI) 1.09–1.82] and cardiovascular mortality (HR 1.49, 95% CI 1.09–2.03), which was driven by a higher risk for fatal myocardial infarction (HR 2.65, 95% CI 1.46–4.81). Loss of Y chromosome > 17% was associated with a profibrotic and proinflammatory plasma protein expression profile as characterized by higher plasma levels of osteoprotegerin, matrix metalloproteinase-12, growth differentiation factor 15, heparin-binding EGF-like growth factor, and resistin. Genetic predisposition for lower myocardial fibrosis attenuated the association between LOY and cardiovascular mortality. Genome-wide methylation analyses identified differential methylation in 298 genes including ACTB, RPS5, WDR1, CD151, and ARAP1. Single-cell RNA sequencing further confirmed differential gene expression of 37 of these genes in LOY in peripheral blood mononuclear cells comprising a set of fibrosis-regulating genes including RPS5. RPS5 silencing in macrophages induced a paracrine induction of collagen expression in cardiac fibroblasts documenting a functional role in vitro.
Conclusions
LOY represents an important independent risk factor for cardiovascular mortality in male patients with coronary artery disease. Targeting LOY may represent a sex-specific personalized medicine approach.