2024-02-24 ワシントン大学セントルイス校(WashU)
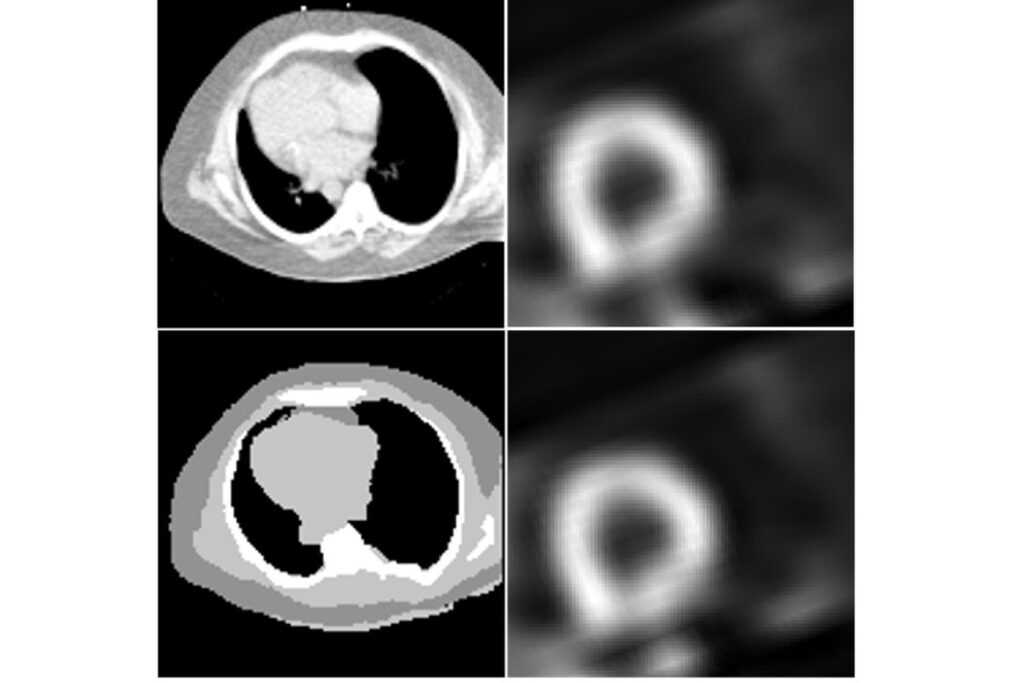 SPECT images (right) and attenuation maps (left) obtained by traditional methods (top) and the new CTLESS method (bottom). (Image: Jha Lab)
SPECT images (right) and attenuation maps (left) obtained by traditional methods (top) and the new CTLESS method (bottom). (Image: Jha Lab)
<関連情報>
- https://source.washu.edu/2025/02/deep-learning-to-increase-accessibility-ease-of-heart-imaging/
- https://engineering.washu.edu/news/2025/Deep-learning-to-increase-accessibility-ease-of-heart-imaging.html
- https://ieeexplore.ieee.org/document/10767308
CTLESS: 心筋灌流SPECTのための散乱窓投影とディープラーニングに基づく透過型減弱補正法 CTLESS: A scatter-window projection and deep learning-based transmission-less attenuation compensation method for myocardial perfusion SPECT
Zitong Yu; Md Ashequr Rahman; Craig K. Abbey; Richard Laforest; Nancy A. Obuchowski; Barry A. Siegel
IEEE Transactions on Medical Imaging Published:25 November 2024
DOI:https://doi.org/10.1109/TMI.2024.3496870
Abstract:
Attenuation compensation (AC), while being beneficial for visual-interpretation tasks in myocardial perfusion imaging (MPI) by single-photon emission computed tomography (SPECT), typically requires the availability of a separate X-ray CT component, leading to additional radiation dose, higher costs, and potentially inaccurate diagnosis in case of misalignment between SPECT and CT images. To address these issues, we developed a method for cardiac SPECT AC using deep learning and emission scatter-window photons without a separate transmission scan (CTLESS). In this method, an estimated attenuation map reconstructed from scatter-energy window projections is segmented into different regions using a multi-channel input multi-decoder network trained on CT scans. Pre-defined attenuation coefficients are assigned to these regions, yielding the attenuation map used for AC. We objectively evaluated this method in a retrospective study with anonymized clinical SPECT/CT stress MPI images on the clinical task of detecting perfusion defects with an anthropomorphic model observer. CTLESS yielded statistically non-inferior performance compared to a CT-based AC (CTAC) method and significantly outperformed a non-AC (NAC) method on this clinical task. Similar results were observed in stratified analyses with different sexes, defect extents, and defect severities. The method was observed to generalize across two SPECT scanners, each with a different camera. In addition, CTLESS yielded similar performance as CTAC and outperformed NAC method on the fidelity-based figures of merit, namely, root mean squared error (RMSE) and structural similarity index measure (SSIM). Moreover, as we reduced the training dataset size, CTLESS yielded relatively stable AUC values and generally outperformed another DL-based AC method that directly estimated the attenuation coefficient within each voxel. These results demonstrate the capability of the CTLESS method for transmission-less AC in SPECT and motivate further clinical evaluation.


