2025-03-14 京都大学
 本研究の概要
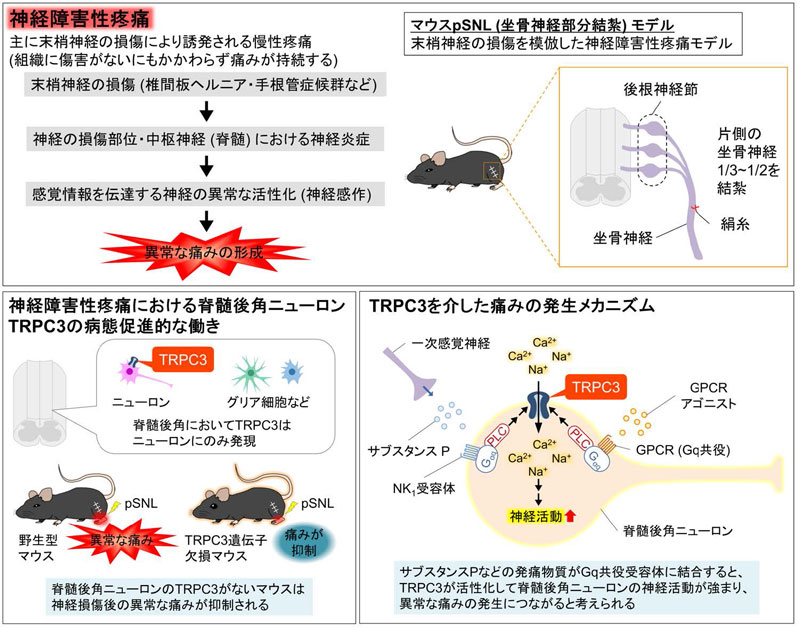
本研究の概要
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-03-14-0
- https://www.kyoto-u.ac.jp/sites/default/files/2025-03/web_2503_Shirakawa-607d2a4ff496ef8bcbd00b70be205017.pdf
- https://www.pnas.org/doi/10.1073/pnas.2416828122
脊髄TRPC3は神経因性疼痛を促進し、ホスホリパーゼCによる機械的過敏症を調整する Spinal TRPC3 promotes neuropathic pain and coordinates phospholipase C–induced mechanical hypersensitivity
Shota Tobori, Kosei Tamada, Nagi Uemura, +6 , and Hisashi Shirakawa
Proceedings of the National Academy of Sciences Published:March 13, 2025
DOI:https://doi.org/10.1073/pnas.2416828122
Significance
Here, we demonstrate the pathophysiological role of TRPC3 in the development of neuropathic pain using TRPC3-knockout mice. TRPC3 deficiency suppressed pain hypersensitivity in a neuropathic pain mouse model. TRPC3 is mainly expressed in the spinal dorsal horn neurons. The knockdown of TRPC3 in spinal dorsal horn neurons alleviates neuropathic pain, whereas its activation induces acute mechanical hypersensitivity. Furthermore, mechanical hypersensitivity induced by phospholipase C, which is associated with the pathology of neuropathic pain, was suppressed in TRPC3-knockout mice. Our findings elucidate the TRPC3-mediated cellular and molecular mechanisms underlying the pathogenesis of neuropathic pain and suggest that TRPC3 may be a therapeutic target for the effective treatment of this condition.
Abstract
Neuropathic pain is a debilitating chronic condition mainly caused by peripheral nerve injury. However, the cellular and molecular mechanisms underlying this condition remain unclear. Transient receptor potential canonical 3 (TRPC3), a TRP channel that is activated by downstream of the Gq-phospholipase C (PLC) axis, is expressed in the somatosensory system. Therefore, the present study investigated its pathophysiological role in neuropathic pain following peripheral nerve injury. Here, partial sciatic nerve ligation (pSNL) elicited mechanical and thermal hypersensitivity in wild-type mice, which was suppressed in TRPC3-KO mice. In situ hybridization revealed that TRPC3 is predominantly expressed in neurons in the spinal dorsal horn. Furthermore, spinal dorsal horn neuron-specific downregulation using miRNA attenuated pSNL-induced mechanical hypersensitivity. Spinal TRPC3 activation elicited acute mechanical hypersensitivity. Moreover, its genetic ablation reduced the mechanical hypersensitivity caused by spinal NK1R or PLC activation. These findings demonstrate that TRPC3 in spinal dorsal horn neurons facilitates the development of neuropathic pain. Therefore, TRPC3 may be a promising therapeutic target for neuropathic pain caused by peripheral nerve injury.