2025-03-17 アメリカ国立衛生研究所 (NIH)
<関連情報>
- https://www.nih.gov/news-events/news-releases/nih-sponsored-trial-lassa-vaccine-opens
- https://www.nature.com/articles/s41541-024-00930-z#Sec2
狂犬病ベースの不活化ラッサ熱ウイルスワクチン候補LASSARABが非ヒト霊長類を致死的疾患から守る Inactivated rabies-based Lassa fever virus vaccine candidate LASSARAB protects nonhuman primates from lethal disease
Gabrielle Scher,Catherine Yankowski,Drishya Kurup,Nicole M. Josleyn,Eric R. Wilkinson,Jay Wells,Jesse Steffens,Ginger Lynn,Sean Vantongeren,Xiankun Zeng,Nancy Twenhafel,Kathleen A. Cashman & Matthias J. Schnell
npj Vaccines Published:09 August 2024
DOI:https://doi.org/10.1038/s41541-024-00930-z
Abstract
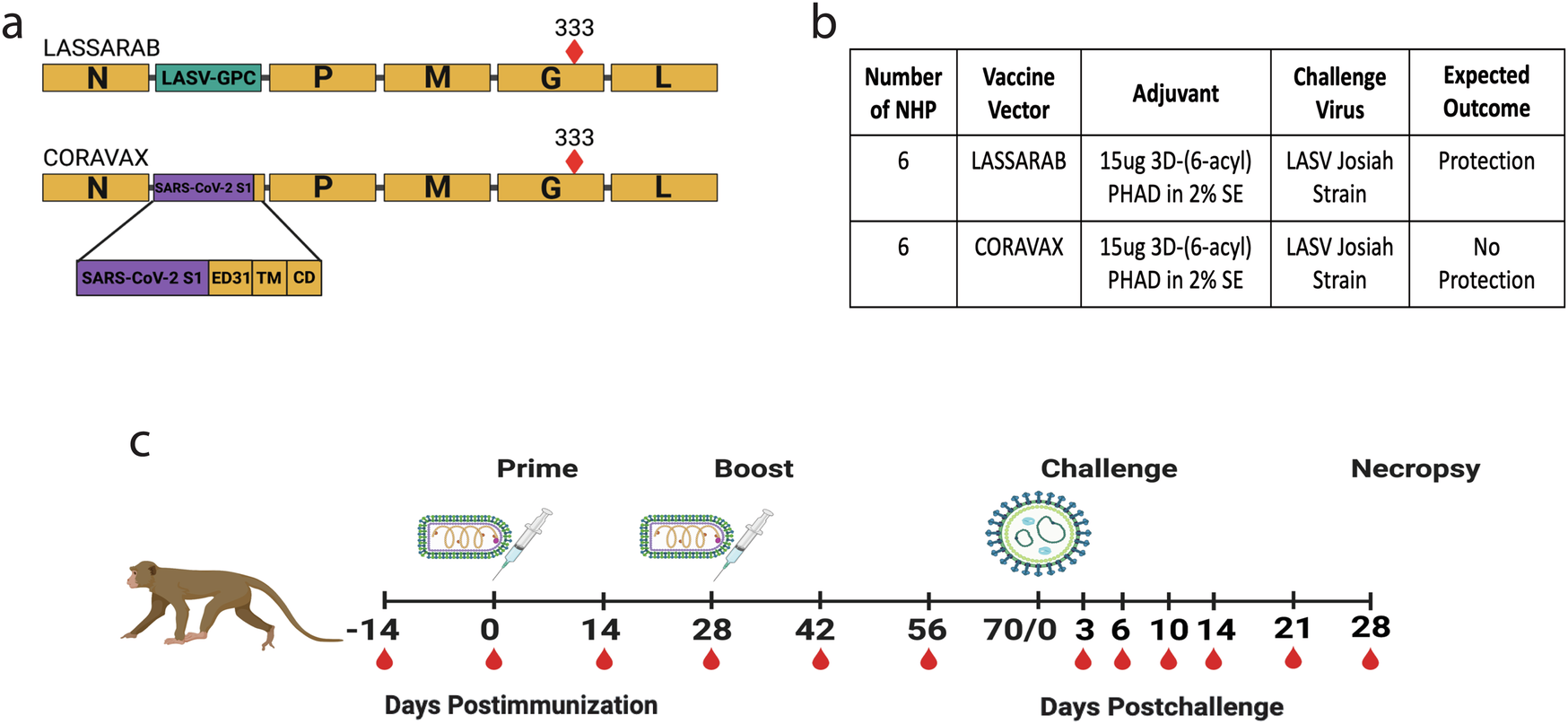
Lassa fever virus (LASV), a member of the Arenavirus family, is the etiological agent of Lassa fever, a severe hemorrhagic disease that causes considerable morbidity and mortality in the endemic areas of West Africa. LASV is a rodent-borne CDC Tier One biological threat agent and is on the World Health Organization’s (WHO) Priority Pathogen list. Currently, no FDA-licensed vaccines or specific therapeutics are available. Here, we describe the efficacy of a deactivated rabies virus (RABV)-based vaccine encoding the glycoprotein precursor (GPC) of LASV (LASSARAB). Nonhuman primates (NHPs) were administered a two-dose regimen of LASSARAB or an irrelevant RABV-based vaccine to serve as a negative control. NHPs immunized with LASSARAB developed strong humoral responses to LASV-GPC. Upon challenge, NHPs vaccinated with LASSARAB survived to the study endpoint, whereas NHPs in the control group did not. This study demonstrates that LASSARAB is a worthy candidate for continued development.

