2025-07-02 アメリカ国立衛生研究所(NIH)
<関連情報>
- https://www.nih.gov/news-events/news-releases/nih-study-links-particulate-air-pollution-increased-mutations-lung-cancers-among-nonsmokers
- https://www.nature.com/articles/s41586-025-09219-0
喫煙経験のない人の肺がんのゲノムを形成する変異原性の力 The mutagenic forces shaping the genomes of lung cancer in never smokers
Marcos Díaz-Gay,Tongwu Zhang,Phuc H. Hoang,Charles Leduc,Marina K. Baine,William D. Travis,Lynette M. Sholl,Philippe Joubert,Azhar Khandekar,Wei Zhao,Christopher D. Steele,Burçak Otlu,Shuvro P. Nandi,Raviteja Vangara,Erik N. Bergstrom,Mariya Kazachkova,Oriol Pich,Charles Swanton,Chao Agnes Hsiung,I-Shou Chang,Maria Pik Wong,Kin Chung Leung,Jian Sang,John P. McElderry,… Maria Teresa Landi
Nature Published:02 July 2025
DOI:https://doi.org/10.1038/s41586-025-09219-0
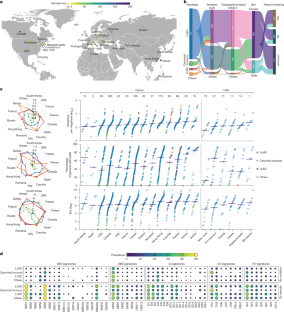
Abstract
Lung cancer in never smokers (LCINS) accounts for around 25% of all lung cancers1,2 and has been associated with exposure to second-hand tobacco smoke and air pollution in observational studies3,4,5. Here we use data from the Sherlock-Lung study to evaluate mutagenic exposures in LCINS by examining the cancer genomes of 871 treatment-naive individuals with lung cancer who had never smoked, from 28 geographical locations. KRAS mutations were 3.8 times more common in adenocarcinomas of never smokers from North America and Europe than in those from East Asia, whereas a higher prevalence of EGFR and TP53 mutations was observed in adenocarcinomas of never smokers from East Asia. Signature SBS40a, with unknown cause6, contributed the largest proportion of single base substitutions in adenocarcinomas, and was enriched in cases with EGFR mutations. Signature SBS22a, which is associated with exposure to aristolochic acid7,8, was observed almost exclusively in patients from Taiwan. Exposure to secondhand smoke was not associated with individual driver mutations or mutational signatures. By contrast, patients from regions with high levels of air pollution were more likely to have TP53 mutations and shorter telomeres. They also exhibited an increase in most types of mutations, including a 3.9-fold increase in signature SBS4, which has previously been linked with tobacco smoking9, and a 76% increase in the clock-like10 signature SBS5. A positive dose–response effect was observed with air-pollution levels, correlating with both a decrease in telomere length and an increase in somatic mutations, mainly attributed to signatures SBS4 and SBS5. Our results elucidate the diversity of mutational processes shaping the genomic landscape of lung cancer in never smokers.